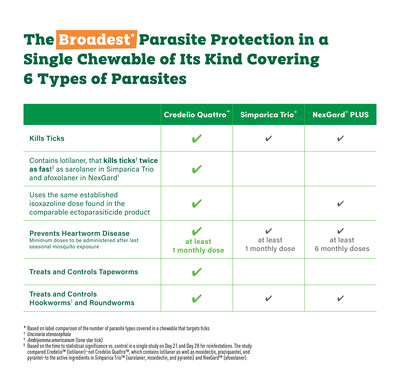
Now Available: Credelio Quattro™ (lotilaner, moxidectin, praziquantel, and pyrantel chewable tablets) Provides Broadest[i] Parasite Protection of Its Kind
- Six-in-One Parasite Protection in One Chewable Tablet
- Six of Six Potential Blockbusters from Elanco Now In-Market
GREENFIELD, Ind., Jan. 23, 2025 /PRNewswire/ -- Elanco Animal Health Incorporated (NYSE:ELAN) today announced Credelio Quattro™ (lotilaner, moxidectin, praziquantel, and pyrantel chewable tablets) is now available for veterinarians to order at CredelioQuattroVet.com. With four established and powerful ingredients, Credelio Quattro is designed to deliver the broadest i parasite protection of its kind. Credelio Quattro is the first and only monthly chewable tablet of its kind to protect against six different types of parasites, including three risky intestinal worms that can be passed to humans:
- Tapeworms
- Roundworms
- Hookwormsii
- Heartworms
- Ticks
- Fleas
"Today is a historic day as we bring veterinarians and pet owners peace of mind with the broadest i parasite protection of its kind for dogs," said Bobby Modi, Executive Vice President, U.S. Pet Health and Global Digital Transformation. "Our research shows 94% of dog owners believe that proactive treatment for intestinal worms, including tapeworms, is a top priority. And when looking for intestinal worm protection for their dog, owners care most about product effectiveness, parasite coverage and duration of protection. By providing the broadesti range of parasite protection of its kind, covering six parasites in a single, chewable, Credelio Quattro delivers on pet owner expectations, while strengthening Elanco's market presence and positioning us for continued growth in the parasiticide segment."
But the survey shows an important gap where pet owners need to lean in to better protect their beloved pets against risky parasites. Just one in three respondents believe that fleas and ticks are a risk year-round; and only half of dog owners surveyed protect against heartworm disease year-round.iii With the geographic spread of parasites and increasing disease pressure, the importance of year-round protection is more significant than ever.
Broad. Fast. Tasty. Tough.
The broad range of parasite protection from Credelio Quattro in a single, chewable monthly tablet makes it easy for pet parents to protect against some of the most common and challenging parasites. According to studies, Credelio Quattro has been shown to be 100% efficacious against tapeworms with its industry-established dose of praziquantel (one of the active ingredients found in Credelio Quattro).iv Credelio Quattro also contains lotilaner that kills ticksv twice as fastvi as sarolaner in Simparica Trio® and afoxolaner in NexGard®.vii
"Parasites like tapeworms, fleas and ticks can carry dangerous diseases and some of these can spread from dogs to humans," said Dr. Casey Locklear, veterinarian and parasiticide lead for Elanco Animal Health. "The Companion Animal Parasite Council (CAPC) recommends year round prevention of parasites, even during the winter months and specifically mentions the need for monthly deworming of dogs with praziquantel in areas where a certain type of tapeworm – E. granulosus tapeworms – are endemic."viii
Veterinarians can place their orders today at CredelioQuattroVet.com. Credelio Quattro will make its debut at the Veterinary Meeting & Expo (VMX) in Orlando from January 25-29, 2025. Attendees can visit Booth #4617 to place their orders, learn more and speak with experts on-site.
Launching Credelio Quattro is another milestone in Elanco's historic era of innovation and delivery as it is one of six potential blockbuster products from Elanco available in the U.S.
ABOUT ELANCO
Elanco Animal Health Incorporated (NYSE:ELAN) is a global leader in animal health dedicated to innovating and delivering products and services to prevent and treat disease in farm animals and pets, creating value for farmers, pet owners, veterinarians, stakeholders and society as a whole. With 70 years of animal health heritage, we are committed to breaking boundaries and going beyond to help our customers improve the health of animals in their care, while also making a meaningful impact on our local and global communities. At Elanco, we are driven by our vision of Food and Companionship Enriching Life and our Elanco Healthy Purpose™ sustainability pillars – all to advance the health of animals, people, the planet and our enterprise. Learn more at www.elanco.com.
Indications: Credelio Quattro is indicated for the prevention of heartworm disease and the treatment and control of roundworm, hookworm* and tapeworm infections. Credelio Quattro kills adult fleas and is indicated for the treatment and prevention of flea infestations and the treatment and control of tick infestations for 1 month in dogs and puppies 8 weeks of age and older and weighing 3.3 pounds or greater.
Important Safety Information: Lotilaner, an ingredient in Credelio Quattro, belongs to the isoxazoline class and has been associated with neurologic adverse reactions like tremors, ataxia, and seizures even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders. Dogs should be tested for existing heartworm infections before Credelio Quattro administration as it is not effective against adult D. immitis. The safe use in breeding, pregnant, or lactating dogs has not been evaluated. The most frequently reported adverse reactions in clinical trials were vomiting and diarrhea. For complete safety information, please see Credelio Quattro product label or ask your veterinarian.
*Uncinaria stenocephala
NexGard is a registered trademark of Boehringer Ingelheim Animal Health USA Inc. Simparica TRIO is a registered trademark of Zoetis Services LLC.
Credelio Quattro, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates. Other company and product names are trademarks of their respective owners. © 2025 Elanco or its affiliates.
____________________ |
i Based on label comparison of the number of parasite types covered in a chewable that covers targets ticks. |
ii Uncinaria stenocephala |
iii Elanco Animal Health, Data on File. February 2024. |
iv Echinococcus granulosus, Dipylidium caninum, and Taenia pisiformis |
v Amblyomma americanum (lone star tick) |
vi Based on the time to statistical significance vs control in a single study on Day 21 and Day 28 for reinfestations. The study compared Credelio™ (lotilaner)—not Credelio Quattro™, which contains lotilaner as well as moxidectin, praziquantel, and pyrantel—to the active ingredients in Simparica Trio™ (sarolaner, moxidectin, and pyrantel) and NexGard™ (afoxolaner). |
vii Reif KE, Kollasch TM, Neilson JC, Herrin BH, Ryan WG, Bell MC, Beltz MS, Dryden MW, Jesudoss Chelladurai JRJ, Miller KR, Sutherland CJ. Comparative speed of kill provided by lotilaner (Credelio™), sarolaner (Simparica Trio™), and afoxolaner (NexGard™) to control Amblyomma americanum infestations on dogs. Parasite Vectors. 2024 Jul 20;17(1):313. |
viii Echinococcus spp. Companion Animal Parasite Council Guidelines. Updated Sept 13, 2022. Accessed Oct 10, 2024. https://capcvet.org/guidelines/echinococcus-spp/. |
Investor Contact: Tiffany Kanaga (765) 740-0314 [email protected]
Media Contact: Season Solorio (765) 316-0233 [email protected]
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/now-available-credelio-quattro-lotilaner-moxidectin-praziquantel-and-pyrantel-chewable-tablets-provides-broadesti-parasite-protection-of-its-kind-302357947.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/now-available-credelio-quattro-lotilaner-moxidectin-praziquantel-and-pyrantel-chewable-tablets-provides-broadesti-parasite-protection-of-its-kind-302357947.html
SOURCE Elanco Animal Health