Abbott's Dissolving Stent Receives Health Canada Approval for Treating Blocked Arteries Below-the-Knee
- More than 800,000 Canadians1 live with peripheral artery disease (PAD) with limited treatment options to date1
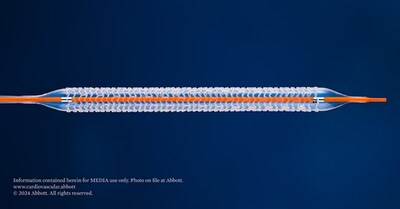
- The first-of-its-kind dissolvable stent, the Esprit™ BTK Everolimus Eluting Resorbable Scaffold, dissolves over time after it has opened blocked arteries below-the-knee (BTK)
- The dissolving stent offers the possibility of better outcomes for people with the most severe form of PAD
TORONTO, Sept. 29, 2025 /CNW/ -- Abbott (NYSE: ABT) today announced that Health Canada has authorized the Esprit™ BTK Everolimus Eluting Resorbable Scaffold System (Esprit BTK System), a first-of-its kind treatment innovation for people with chronic limb threatening ischemia (CLTI) below-the-knee (BTK). The Esprit BTK System is designed to keep arteries open and deliver a drug, everolimus, to support vessel healing prior to completely dissolving.
Until this recent Health Canada approval for the Esprit BTK System, the standard of care has been balloon angioplasty, which relies on a small balloon delivered via a catheter to the blockage to compress it against the arterial wall, opening the vessel and restoring blood flow. However, blockages treated only with balloon angioplasty can have poor short-and long-term results, and in many instances, the vessels become blocked again, requiring additional treatment.
The Esprit BTK System is a first-of-its-kind dissolvable stent and is comprised of material similar to dissolving sutures. The device is implanted during a catheter-based minimally invasive procedure via a small incision in the leg. Once the blockage is open, the Esprit BTK System helps heal the vessel and provides support for approximately three years until the vessel is strong enough to remain open on its own.
In November 2024 at the Vascular InterVentional Advances (VIVA) Conference in Las Vegas, Abbott released late-breaking clinical data on the LIFE-BTK trial two-year results.4 The trial demonstrated that compared to balloon angioplasty, the Esprit BTK System results in improved patient outcomes and 48% fewer repeat procedures over the study period.
"Health Canada's approval of Abbott's Esprit BTK System marks a significant milestone in our fight against peripheral artery disease below-the-knee and represents a new era of improved outcomes for people worldwide," said Brian DeRubertis, MD, FACS, New York Presbyterian-Weill Cornell Medical Center and one of the principal investigators of the LIFE-BTK trial. "Abbott is changing the landscape of CLTI therapy by introducing a treatment option that is superior to balloon angioplasty."
Peripheral Artery Disease (PAD) is highly prevalent, yet many people have never heard of the condition.5 More than 800,000 Canadians are living with this painful disease and only a small percentage of those people have been diagnosed.1 CLTI is a serious form of PAD that occurs when arteries become clogged with plaque, preventing blood flow and oxygen from reaching the lower leg and foot. People living with CLTI often experience extreme pain and open wounds that do not heal. One in five CLTI patients require an amputation, with over 50% of those having never had any vascular investigation and 60% of the amputations result in mortality within 5 years.2,3
"At Abbott, we've recognized the significant burden of disease and limited treatment options for people living with the most severe form of PAD – treatments that typically include high-risk surgery or less invasive options that come with limitations," said Julie Tyler, senior vice president of Abbott's vascular business. "Our revolutionary below-the-knee resorbable scaffold technology meets an unmet need that will ultimately help people with PAD live better and fuller lives."
As part of Abbott's continued commitment to helping all people live healthier lives, PAD and CLTI education information can be found at www.PAD-info.com. Physicians can find more information at CLEAR Program | Abbott.
For important safety information on the Esprit BTK System, visit: Esprit™ BTK System - Canada
Link to b-roll: Abbott's Esprit BTK Dissolving Stent
About Abbott:
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 114,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com and on LinkedIn, Facebook, Instagram, X and YouTube.
SOURCES
1 Bonneau C, Caron NR, Hussain MA, Kayssi A, Verma S et Al-Omran M (2018). Peripheral artery disease among Indigenous Canadians: What do we know? Canadian journal of surgery. Journal canadien de chirurgie, 61(5), 305-310. https://doi.org/10.1503/cjs.013917.
2 Li J, Varcoe R, Manzi M, Kum S, Iida O, Schmidt A, Shishehbor MH. Below-the-Knee Endovascular Revascularization: A Position Statement. JACC: Cardiovascular Interventions. 2024; ISSN 1936-8798
3 McGinigle KL, Minc SD. Disparities in amputation in patients with peripheral arterial disease. Surgery. Juin 2021; 169(6): 1290-1294. doi:10.1016/j.surg.2021.01.025.
4 Brian G. DeRubertis, et al. Two-Year Outcomes of the LIFE-BTK Randomized Controlled Trial Evaluating the Esprit™ BTK Drug-eluting Resorbable Scaffold for Treatment of Infrapopliteal Lesions. VIVA 2024.
5 Grant E (8 février 2025). New data: 70% of Americans unaware of common vascular disease that is one of the leading causes of amputation. Society of Interventional Radiology. https://www.sirweb.org/for-press/new-data-70-of-americans-unaware-of-common-vascular-disease-that-is-one-of-the-leading-causes-of-amputation/.
SOURCE Abbott

![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/September2025/29/c4951.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/September2025/29/c4951.html