Actinium Pharmaceuticals Presents New Data Demonstrating Potent and Durable Efficacy of ATNM-400, a First-in-Class Multi-Tumor Actinium-225 Radiotherapy, at the 32nd Annual Prostate Cancer Foundation Scientific Retreat
- ATNM-400 targets a novel, non-PSMA antigen implicated in disease biology with
continued expression following androgen receptor pathway inhibitor (ARPI) therapy
and 177Lu-PSMA-617 therapy, enabling potent activity independent of PSMA
expression
- ATNM-400 demonstrated superior efficacy over PSMA-targeted radioligand therapy
(177Lu-PSMA-617 and 225Ac-PSMA-617) with enhanced tumor control and
prolonged survival in low-PSMA and treatment-resistant models
- Demonstrated durable anti-tumor responses, exceeding those achieved with
the ARPI enzalutamide
- Combination of ATNM-400 with enzalutamide achieved complete tumor regression
in 40% of prostate cancer-bearing animals, demonstrating strong mechanistic
synergy with AR pathway inhibition
NEW YORK, Oct. 24, 2025 /PRNewswire/ -- Actinium Pharmaceuticals, Inc. (NYSE:ATNM) (Actinium or the Company), a pioneer in the development of differentiated targeted radiotherapies, today highlighted new preclinical data for ATNM-400, its novel, first-in-class antibody radioconjugate armed with the potent alpha-emitter Actinium-225 (Ac-225) at the 32nd Annual Prostate Cancer Foundation (PCF) Scientific Retreat being held October 23 – 25, 2025 in Carlsbad, CA.
ATNM-400, which targets a non-PSMA antigen associated with prostate cancer progression and treatment resistance, demonstrated superior tumor control and improved overall survival compared with the active agents in key standard-of-care therapies including enzalutamide (the active agent in Xtandi®, Astellas/Pfizer) and 177Lu-PSMA-617 (the active agent in Pluvicto®, Novartis), as well as 225Ac-PSMA-617 across multiple preclinical prostate cancer models.
These data further reinforce ATNM-400's potential as a paradigm changing, PSMA-independent Ac-225 alpha radiotherapy, offering opportunities for use as a monotherapy, in combination regimens, and in patients relapsing after ARPI or PSMA-directed treatments. ATNM-400 data has now been presented at the American Association for Cancer Research (AACR), Society for Nuclear Medicine and Molecular Imaging (SNMMI) and PCF annual conferences in 2025 demonstrating its growing potential in various treatment settings in prostate cancer. In addition, data highlighting its potential in non-small cell lung cancer will be presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics on Saturday, October 25, 2025.
Key Findings in Prostate Cancer Treatment Settings:
Potent and Durable Tumor Control, Increased Overall Survival and Synergistic Activity in Enzalutamide Resistant Prostate Cancer
- Studies were performed in 22Rv1 prostate cancer models, which are known to be resistant to enzalutamide
- ATNM-400 demonstrated superior and 5-times more durable anti-tumor efficacy extending to 100 days compared to approximately 20 days with enzalutamide alone
- Given that ARPI therapies like enzalutamide are known to increase the ATNM-400 target antigen expression, combination treatment with ATNM-400 and enzalutamide was synergistic, resulting in complete tumor eradication in 40% of treated animals and significantly prolonged survival, whereas enzalutamide alone provided no durable disease control
- In models that progressed on enzalutamide, subsequent treatment with ATNM-400 achieved robust tumor control and extended survival, highlighting its potential in post-enzalutamide settings
Highly Effective and Increased Overall Survival in 177Lu-PSMA-617 Resistant Prostate Cancer
- 22Rv1 prostate cancer models are also resistant to 177Lu-PSMA-617
- ATNM-400 overcame 177Lu-PSMA-617 resistance with approximately 5-times long tumor control and 2-times longer overall survival in tumor bearing animals
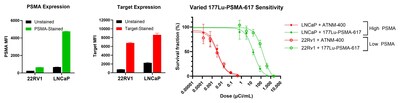
Potent Therapeutic Activity Independent of PSMA Expression Levels
- ATNM-400 targets a highly differentiated, non-PSMA antigen associated with the development and progression of prostate cancer, exhibiting potent therapeutic activity independent of PSMA expression levels
- The expression of the ATNM-400 antigen target was not altered post-177Lu-PSMA-617
- 22Rv1 cell lines are low-PSMA expressing and LNCaP cell lines are high-PSMA expressing
Addressing Critical Unmet Needs in mCRPC
Prostate cancer patients who progress to mCRPC face limited treatment options. While ARPI therapies like enzalutamide and PSMA-targeted radiotherapies like Pluvicto® have extended survival, resistance and disease progression remain major challenges. In addition, a significant number of patients do not respond to PSMA-targeted radiotherapy as approximately 25%-30% of patients with mCRPC have low or no detectable PSMA and approximately 60% of patients have at least one PSMA-negative prostate cancer lesion.
By targeting a distinct, non-PSMA antigen associated with treatment resistance and poor outcomes, ATNM-400 represents a mechanistically differentiated alpha-radiotherapy approach that has potential in various treatment settings in prostate cancer. Its combination of a high-affinity antibody and potent alpha-emitting Ac-225 payload provides a path to overcome therapeutic resistance and extend patient survival beyond current standards of care.
Differentiation and Potential Clinical Impact
Sandesh Seth, Chairman and Chief Executive Officer, Actinium Pharmaceuticals, Inc., stated, "Since unveiling ATNM-400 earlier this year at AACR, our enthusiasm for this program only continues to grow commensurate with the promising results generated supporting its potential in various treatment settings in prostate cancer. ATNM-400 combines the precision of antibody targeting with the unparalleled potency of Ac-225 alpha particles, and importantly, it does so through a biologically distinct, PSMA-independent mechanism. These new preclinical data presented at PCF provide further validation for ATNM-400 and positions it as a candidate capable of transforming the treatment paradigm for patients with mCRPC. We are thrilled by the positive reactions to our ATNM-400 data and its differentiated non-PSMA approach."
Mr. Seth added, "As we have recently announced, beyond prostate cancer, we are also advancing ATNM-400 into non-small cell lung cancer (NSCLC), where it has shown the ability to overcome resistance to osimertinib (TAGRISSO®, AstraZeneca) an EGFR-TKI therapy in preclinical models. Together, these data highlight Actinium's innovate approach to R&D that leverages our radiochemistry expertise and strong translational biology capabilities to create first-in-class, multi-indication radiotherapeutics."
Actinium Pharmaceuticals envisions multiple clinical applications for ATNM-400 in prostate cancer, including:
- Monotherapy in earlier disease settings prior to PSMA-directed radiotherapy.
- Combination therapy with ARPI's to enhance and prolong treatment responses.
- Sequential therapy for patients relapsing after ARPI or PSMA-targeted radioligand treatments.
Summary of Preclinical Highlights of ATNM-400
- Novel Mechanism: First-in-class Ac-225-based antibody radioconjugate against a non-PSMA target, overexpressed in advanced and resistant prostate cancer.
- Superior Efficacy: Outperformed 177Lu-PSMA-617, Ac225-PSMA-617 and enzalutamide in direct head-to-head preclinical comparisons.
- Durable Responses: A single 40 µCi/kg dose produced strong tumor inhibition; multi-dose regimens yielded long-term tumor control exceeding both enzalutamide and 177Lu-PSMA-617.
- Overcomes Resistance: ATNM-400 maintained potent anti-tumor activity in models resistant to both enzalutamide and 177Lu-PSMA-617.
- Combination Synergy: Synergized with enzalutamide, achieving complete tumor regressions in 40% of treated animals and markedly extending survival.
- Favorable Biodistribution: Demonstrated selective and durable tumor uptake and rapid clearance from normal tissues, supporting a strong therapeutic index.
About ATNM-400
ATNM-400 is a highly innovative, first-in-class, and multi-indication Actinium-225 (Ac-225) targeted radiotherapy candidate in development for prostate cancer and non-small cell lung cancer (NSCLC). ATNM-400 is highly differentiated in prostate cancer as it targets a distinct non-PSMA antigen strongly implicated in prostate cancer progression and treatment resistance. Unlike 177Lu-PSMA-617, the active agent in Pluvicto® and the majority of radiotherapies under development, which rely on PSMA targeting, ATNM-400 is designed to maintain efficacy in low-PSMA or PSMA-resistant disease, a major unmet clinical need. Ac-225 delivers high-linear-energy-transfer alpha particles that induce irreparable double-strand DNA breaks, offering superior potency over beta emitters like Lutetium-177 (177Lu), and has a shorter tissue path length that may reduce off-target toxicity. The receptor specifically targeted by ATNM-400 continues to be expressed at a high level even after androgen receptor inhibitor (ARPI) and ATNM-400 has shown to overcome resistance to the ARPI therapy enzalutamide and work synergistically in combination with enhanced tumor control including complete tumor regression. In NSCLC, ATNM-400 has shown to overcome resistance to osimertinib, an EGFR tyrosine kinase inhibitor (TKI) that is a standard of care therapy.
Prostate cancer is the most commonly diagnosed cancer in men, with ~1.5 million new cases globally and over 313,000 cases expected in the U.S. in 2025. While early-stage disease is typically managed with surgery, radiation, and ARPI therapy, up to 20% of cases progress to mCRPC - a lethal stage with limited treatment options. Targeted radiotherapy is a growing field in prostate cancer, dominated by PSMA-targeting agents like Pluvicto®, which had sales of over $1.3 billion in 2024, yet many patients either lack PSMA expression or develop resistance to Pluvicto®. In the U.S., 40,000 to 60,000 mCRPC patients annually progress after ARPI therapy that as a class generated sales of over $10.0 billion in 2024 including enzalutamide (Xtandi®), which led the ARPI class with sales of over $5.9 billion in 2024, highlighting a significant unmet need. Lung cancer is the leading cause of cancer deaths and there are there are over 200,000 new cases expected in the U.S. in 2025. NSCLC accounts for approximately 85% of all lung cancer cases. The EGFR TKI Osimertinib (TAGRISSO, AstraZeneca) generated sales of $6.6 billion in 2024. Across prostate cancer and NSCLC, there are approximately 500,000 new cases in the U.S. alone.
About Actinium Pharmaceuticals, Inc.
Actinium is a pioneer in the development of targeted radiotherapies intended to meaningfully improve patient outcomes. ATNM-400, Actinium's lead product candidate, is a novel, first-in-class, and multi-indication Actinium-225 (Ac-225) in development for prostate cancer and non-small cell lung cancer (NSCLC). The antigen specifically targeted by ATNM-400 is highly expressed in metastatic castration-resistant prostate cancer (mCRPC), contributes directly to disease progression, poorer survival outcomes, and continues to be expressed at a high level even after androgen receptor inhibitor (ARPI) and Pluvicto® treatment. ATNM-400 is supported by preclinical data demonstrating tumor-specific uptake, higher efficacy than androgen receptor inhibitor enzalutamide (Xtandi®) and 177Lu-PSMA-617 radiotherapy, the active agent in Pluvicto®, durable tumor control and potent efficacy in prostate cancer models resistant to both enzalutamide and 177Lu-PSMA-617. In addition, ATNM-400 has demonstrated synergy with enzalutamide. The data generated to date with ATNM-400 supports its potential to be used either as a monotherapy, in combination or sequenced with other therapies. Actinium's most advanced product candidate in development is Actimab-A, a CD33 targeting therapeutic, that is a potential backbone therapy for acute myeloid leukemia (AML) and other myeloid malignancies leveraging the mutation agnostic alpha-emitter radioisotope payload Ac-225. Actimab-A has demonstrated potential activity in relapsed and refractory acute myeloid leukemia (r/r AML) patients in combination with the chemotherapy CLAG-M including high rates of Complete Remissions (CR) and measurable residual disease (MRD) negativity leading to improved survival outcomes and is being advanced to a Phase 2/3 trial. In addition, Actinium is engaged with the National Cancer Institute (NCI) under a Cooperative Research and Development Agreement (CRADA) for development of Actimab-A in AML and other myeloid malignancies. The first clinical trial under the CRADA will evaluate the triplet combination comprised of Actimab-A, Venetoclax (Abbvie/Roche) an oral Bcl-2 inhibitor and ASTX-727 (Taiho Oncology, an Otsuka holdings company) a novel oral hypomethylating agent (HMA) in frontline acute myeloid leukemia (AML) patients. Additionally, Actinium is developing Actimab-A as a potential pan tumor therapy in combination with PD-1 checkpoint inhibitors including KEYTRUDA® and OPDIVO® by depleting myeloid derived suppressor cells (MDSCs), which represents a potential multi-billion-dollar addressable market. Iomab-ACT, Actinium's next generation conditioning candidate, is being developed with the goal of improving patient access and outcomes for potentially curative cell and gene therapies. Iomab-B is an induction and conditioning agent prior to bone marrow transplant in patients with r/r AML, which Actinium is seeking a potential strategic partner for a Phase 2/3 trial in the U.S. In addition, the company's R&D efforts are primarily focused on advancing several preclinical programs for solid tumor indications. Actinium holds approximately 240 patents and patent applications including several patents related to the manufacture of the isotope Ac-225 in a cyclotron.
For more information, please visit: https://www.actiniumpharma.com/
Forward-Looking Statements
This press release may contain projections or other "forward-looking statements" within the meaning of the "safe-harbor" provisions of the private securities litigation reform act of 1995 regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management's current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium's products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the "SEC"), including without limitation its most recent annual report on form 10-K, subsequent quarterly reports on Forms 10-Q and Forms 8-K, each as amended and supplemented from time to time.
Investors:
[email protected]
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-pharmaceuticals-presents-new-data-demonstrating-potent-and-durable-efficacy-of-atnm-400-a-first-in-class-multi-tumor-actinium-225-radiotherapy-at-the-32nd-annual-prostate-cancer-foundation-scientific-retreat-302593853.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-pharmaceuticals-presents-new-data-demonstrating-potent-and-durable-efficacy-of-atnm-400-a-first-in-class-multi-tumor-actinium-225-radiotherapy-at-the-32nd-annual-prostate-cancer-foundation-scientific-retreat-302593853.html
SOURCE Actinium Pharmaceuticals, Inc.