Actinium Presents First Ever Data Demonstrating Actimab-A in Combination with Leading Menin Inhibitors Leads to Anti-Tumor Control and Potent Leukemic Cell Killing in Preclinical Acute Myeloid Leukemia Models at the 2024 EHA Congress
- Actimab-A enhances dose-dependent acute myeloid leukemia cell death in KMT2A sensitive acute myeloid leukemia blasts in combination with leading menin inhibitors
- Combination with leading menin inhibitor demonstrates acute myeloid leukemia cell death and significant tumor elimination not achieved with monotherapy
- Menin combination expands backbone potential of Actimab-A in acute myeloid leukemia that already includes chemotherapy, venetoclax and FLT3 inhibitors
NEW YORK, June 17, 2024 /PRNewswire/ -- Actinium Pharmaceuticals, Inc. (NYSE:ATNM) (Actinium or the Company), a leader in the development of Antibody Radiation Conjugates (ARCs) and other targeted radiotherapies, today announced that an abstract detailing the first ever preclinical data from the combination of menin inhibitors with Actinium's ARC Actimab-A in acute myeloid leukemia (AML) models was presented at the 2024 European Hematology Association (EHA) Congress held June 13 – 16, 2024, in Madrid, Spain. Actinium studied Actimab-A in combination with the leading menin inhibitors, revumenib (Syndax Pharmaceuticals, Inc.) and ziftomenib (Kura Oncology, Inc.), which are being developed for patients with KMT2A rearrangements and NMP1 mutations, which are present in approximately 10% and 30% of AML patients, respectively.
Actimab-A + Menin inhibitor combination results include:
- Actimab-A as a single agent showed potent in vitro AML cell killing activity in both MV-4-11 and MOLM-13 KMT2A mutant cell lines, compared to the non-radio conjugated CD33 antibody lintuzumab (p<0.0001)
- Actimab-A enhanced AML cell death when combined with both revumenib and ziftomenib at all dose levels in difficult to treat KMT2A mutant AML
- The combination of Actimab-A with leading menin inhibitors triggered an acute increase in AML necrosis and cell death in vivo relative to single agent therapy within 72 hours of dosing
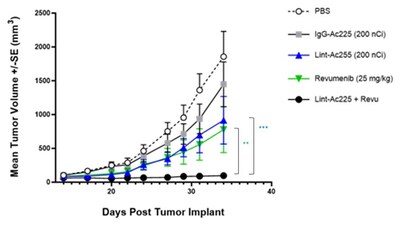
- Anti-tumor effect was significantly potentiated and prolonged when combining Actimab-A with a leading menin inhibitor compared to monotherapies in xenograft leukemia models in vivo (p<0.0024 Actimab-A + menin) as shown in the exhibit below
The Actimab-A + Menin Inhibitor combination presentation can be accessed on the investor relations page of Actinium's website here.
Actimab-A targets CD33, a marker expressed ubiquitously in patients with AML, and is conjugated with the alpha-partible payload Actinium-225. The broad expression of CD33 and the differentiated mutation agnostic cell-killing mechanism of targeted radiotherapy make Actimab-A broadly applicable for combinations with chemotherapy, targeted agents including venetoclax, FLT3 and menin inhibitors, immunotherapies and cellular therapies supporting its potential backbone therapy profile across the AML patient treatment journey.
Sandesh Seth, Actinium's Chairman and CEO, said, "Combining with menin inhibitors is an exciting expansion of the already broad potential of Actimab-A in AML. Across single agent and combination studies, Actimab-A has produced high rates of response, MRD negativity and improved survival in high-risk, relapsed and refractory patients including those with a TP53 mutation and venetoclax failures. The broad expression of CD33 in AML coupled with the potency of Actinium-225 make Actimab-A an ideal agent for treating radiation sensitive AML. We are encouraged by this highly promising initial data and the synergistic potential of Actimab-A with menin inhibitors, which has broad potential across the AML treatment continuum including frontline, maintenance and relapsed/refractory settings. We are eager to continue to study this combination and generate additional data that could support advancing into clinical studies of Actimab-A with menin inhibitors."
Menin inhibitors are a class of drug candidates being developed for patients with AML that have a rearrangement of the KMT2A gene, previously known as the mixed-lineage leukemia (MLL) or mutation of the NPM1 gene. There are multiple menin inhibitors in development for these patients with revumenib (Syndax Pharmaceuticals, Inc.) being most advanced having a PDUFA data of September 2024 and ziftomenib (Kura Oncology, Inc.) enrolling patients in a registration Phase 2 trial. Multiple menin inhibitors are being studied in Phase 1 clinical trials by companies including Johnson & Johnson, Sumitomo Pharma Co., Ltd., Hutchmed, Biomea Fusion, Inc. and BioNova Pharmaceuticals Pvt Ltd.
About Actinium Pharmaceuticals, Inc.
Actinium develops targeted radiotherapies to meaningfully improve survival for people who have failed existing oncology therapies. Advanced pipeline candidates Iomab-B (pre-BLA & MAA (EU)), an induction and conditioning agent prior to bone marrow transplant, and Actimab-A (National Cancer Institute CRADA pivotal development path), a therapeutic agent, have demonstrated potential to extend survival outcomes for people with relapsed and refractory acute myeloid leukemia. Actinium plans to advance Iomab-B for other blood cancers and next generation conditioning candidate Iomab-ACT to improve cell and gene therapy outcomes. Actinium holds more than 230 patents and patent applications including several patents related to the manufacture of the isotope Ac-225 in a cyclotron.
For more information, please visit: https://www.actiniumpharma.com/
Forward-Looking Statements
This press release may contain projections or other "forward-looking statements" within the meaning of the "safe-harbor" provisions of the private securities litigation reform act of 1995 regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management's current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium's products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the "SEC"), including without limitation its most recent annual report on form 10-K, subsequent quarterly reports on Forms 10-Q and Forms 8-K, each as amended and supplemented from time to time.
Investors:
[email protected]
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-presents-first-ever-data-demonstrating-actimab-a-in-combination-with-leading-menin-inhibitors-leads-to-anti-tumor-control-and-potent-leukemic-cell-killing-in-preclinical-acute-myeloid-leukemia-models-at-the-2024-eha-congr-302173741.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-presents-first-ever-data-demonstrating-actimab-a-in-combination-with-leading-menin-inhibitors-leads-to-anti-tumor-control-and-potent-leukemic-cell-killing-in-preclinical-acute-myeloid-leukemia-models-at-the-2024-eha-congr-302173741.html
SOURCE Actinium Pharmaceuticals, Inc.