Anavex Life Sciences Announces Continued Long-Term Benefit from Oral Blarcamesine Compared to Decline Observed in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Control Group
ADAS-Cog13 difference −12.78 (P < 0.0001) with oral blarcamesine treatment compared to ADNI control group at Week 144
77.4 Weeks (17.8 Months) ‘time saved' with oral blarcamesine compared to ADNI
Restoring impaired Autophagy − preceding amyloid-beta and tau
NEW YORK, Oct. 29, 2025 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp. ("Anavex" or the "Company") (NASDAQ:AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer's disease, Parkinson's disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome, and other central nervous system (CNS) disorders, today announced new findings for blarcamesine, an oral small molecule for the potential treatment of early Alzheimer's disease.
New data demonstrate continued long-term benefit from oral blarcamesine compared to decline observed in the Alzheimer's Disease Neuroimaging Initiative (ADNI)1 control group.
Externally matched control participants from the ADNI database were compared with participants over the 144-week period of ANAVEX2-73-AD-004 and its ATTENTION-AD (ANAVEX2-73-AD-EP-004) open-label extension (OLE) Phase IIb/III trial.2 For ADAS-Cog13, total score ranges from 0 to 85 with higher scores indicating increased cognitive impairment.
In the intent-to-treat (ITT) population, significantly less cognitive decline was observed for the blarcamesine participants compared to the ADNI control group at 48 weeks with a significant, and clinically meaningful difference in mean change from baseline ADAS-Cog13 total score of −2.68 points (p < 0.0001).3
Over the course of the open-label extension study at time point 96 weeks, these two groups diverged sharply, with statistically significant differences in mean change in ADAS-Cog13 total score at 96 weeks of −6.41 points (p < 0.0001). The difference between groups continues to increase at 144 weeks (ADAS-Cog13 total score difference of −12.78 points; p < 0.0001).
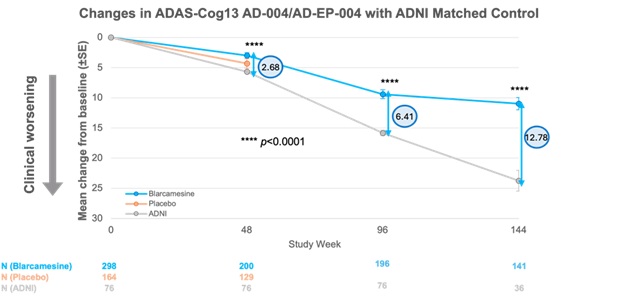
The results provide evidence of the significant beneficial therapeutic effect of blarcamesine, which positively separates from the ADNI control group with duration of treatment.
Alzheimer's disease ‘time saved'
In Alzheimer's disease clinical trials, ‘time saved' refers to the estimated amount of time a treatment delays the progression of the disease, allowing patients to maintain functionality and independence longer. This approach provides a clinically meaningful measure, as it directly relates to the impact on a patient's daily life.4,5 Additionally, blarcamesine exhibited a favorable safety profile with no treatment-related deaths.
In the Phase IIb/III oral blarcamesine early Alzheimer's disease trial, oral blarcamesine resulted in 77.4 weeks (approximately 17.8 months) of time saved in the ITT population compared to the ADNI control group. This measure provides a meaningful clinical perspective, emphasizing the real-world impact of treatment on patients' daily lives and highlighting the potential for long-term therapeutic benefit.
Restoring impaired autophagy
The clinical trial data on blarcamesine also confirmed the upstream mechanism of blarcamesine, restoring impaired autophagy as an early event, preceding amyloid-beta and tau.
The mechanistic confirmation that blarcamesine restores impaired autophagy through SIGMAR1 activation by acting upstream of amyloid and tau pathologies at the molecular level was previously established both in vitro and in vivo. Specifically, studies demonstrated enhanced autophagic flux in human cells and in C. elegans as well as increased proteostasis capacity, ultimately ameliorating paralysis caused by protein aggregation in C. elegans.6
"We remain excited about these enhanced clinically meaningful improvements and accompanied by blarcamesine's favorable safety profile," said Juan Carlos Lopez-Talavera, MD, PhD, Head of Research and Development of Anavex. "Convenient once-daily oral dosing of blarcamesine may allow us to offer a scalable and patient friendly oral pill administration option to patients with early Alzheimer's."
"We are inspired to advancing science across this devastating chronic disease. Alzheimer's disease, like other chronic progressive diseases, requires a long-term therapeutic strategy. Blarcamesine with its convenient once daily oral dosing may lead to greater clinical benefit as evidenced by the significant improvement versus ADNI control group.," said Christopher U Missling, PhD, President and Chief Executive Officer of Anavex. "Additionally, this could help reduce crucial barriers within the currently complex healthcare ecosystem for Alzheimer's disease and potentially provide broader access to a diverse population with early Alzheimer's disease."
Anavex plans to publish and present this new data at international Alzheimer's disease conferences.
This release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that any investigational uses of such product will successfully complete clinical development or gain health authority approval.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (NASDAQ:AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer's disease, Parkinson's disease, schizophrenia, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer. Anavex's lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a and a Phase 2b/3 clinical trial for Alzheimer's disease, a Phase 2 proof-of-concept study in Parkinson's disease dementia, and both a Phase 2 and a Phase 3 study in adult patients and one Phase 2/3 study in pediatric patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate designed to restore cellular homeostasis by targeting SIGMAR1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer's disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson's Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 for the treatment of Parkinson's disease. We believe that ANAVEX®3-71, which targets SIGMAR1 and M1 muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer's disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid, and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the Company on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company's most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: [email protected]
Investors:
Andrew J. Barwicki
Investor Relations
Tel: 516-662-9461
Email: [email protected]
1 Alzheimer's Disease Neuroimaging Initiative (ADNI) is a clinical research project launched by NIH in 2004 to develop methods to predict the onset and progression of Alzheimer's disease and to confirm the effectiveness of treatments. The project involves a multi-year longitudinal observation targeting healthy elderly individuals as well as patients with mild cognitive impairment (MCI) and early stages of Alzheimer's disease.
2 Observed raw data was used. Scheduled visits were [OLE Week 0 = Combined Week 48], [OLE Week 48 = Combined Week 96], [OLE Week 96 = Combined Week 144]; Combined = DB (double-blind) + OLE (open-label-extension) trials. Up to 144 weeks ADNI control group data was available.
3 ADAS-Cog13 scores LS mean difference between the treatment groups being larger than 2 points are considered clinically meaningful improvements: Muir RT, Hill MD, Black SE, Smith EE. Minimal clinically important difference in Alzheimer's disease: Rapid review. Alzheimers Dement. 2024;20(5):3352-3363.
4 Petersen, R C et al. "Expectations and clinical meaningfulness of randomized controlled trials." Alzheimer's & dementia: the journal of the Alzheimer's Association vol. 19,6 (2023): 2730-2736.
5 Dickson, S P et al. ""Time Saved" Calculations to Improve Decision-Making in Progressive Disease Studies." The journal of prevention of Alzheimer's disease. vol. 11,3 (2024): 529-536.
6 Christ, M G et al. "Sigma-1 Receptor Activation Induces Autophagy and Increases Proteostasis Capacity In Vitro and In Vivo." Cells vol. 8,3 211. 2 Mar. 2019.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f19a8c2c-6ef7-43f8-b9dd-771d15db5d15

