Annovis Completes Full Patent Transfer to Crystal Buntanetap
Annovis achieves comprehensive intellectual property (IP) protection now covering both the original semi-crystalline and new crystalline forms of buntanetap.
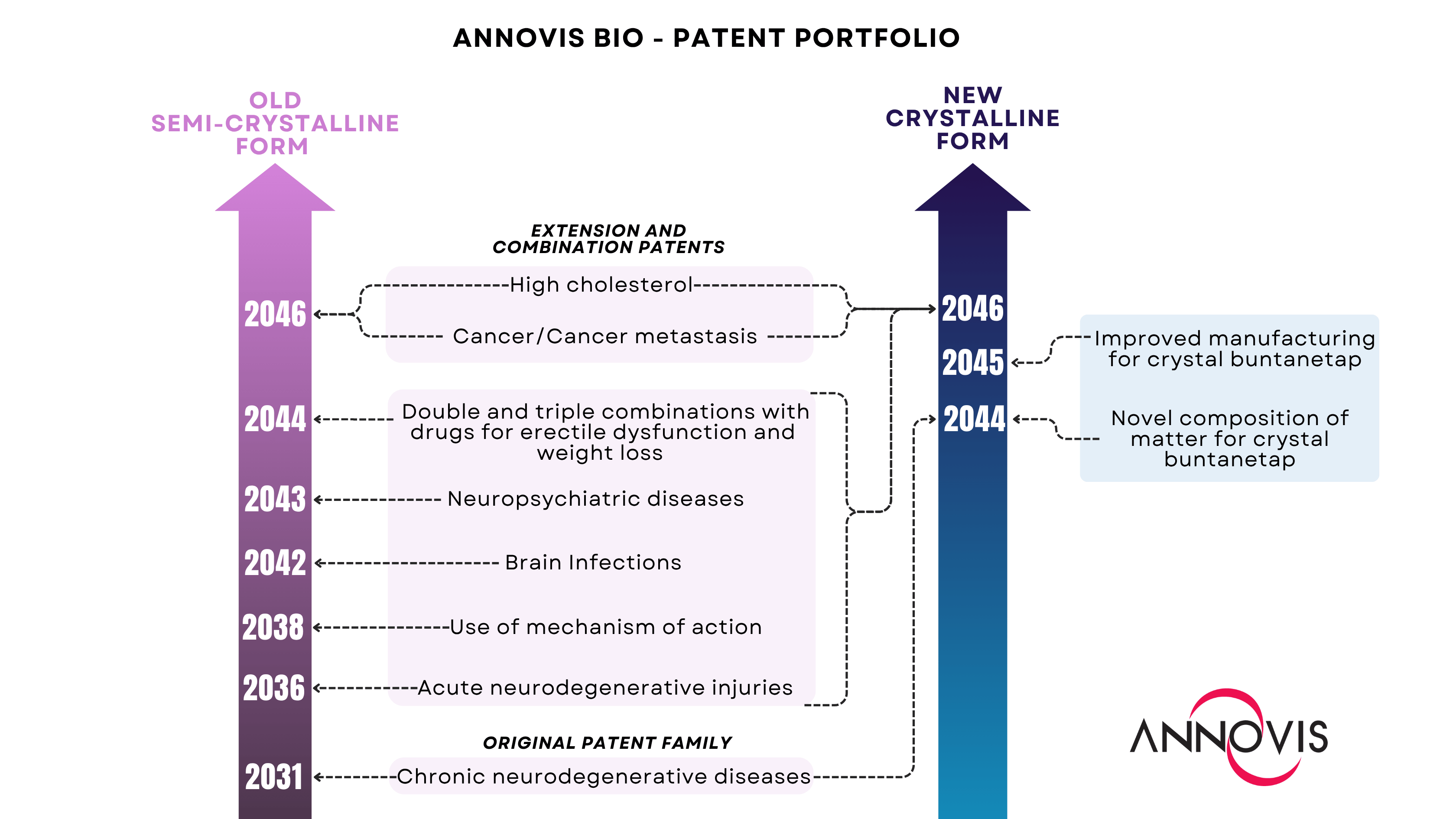
Both forms are covered by a total of 13 patent families.
All patents are filed internationally, securing global coverage of buntanetap.
MALVERN, Pa., Aug. 07, 2025 (GLOBE NEWSWIRE) -- Annovis Bio, Inc. (NYSE:ANVS) ("Annovis" or the "Company"), a late-stage clinical drug platform company pioneering transformative therapies for neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD), today announced that all Company's patents have been successfully transferred from the original form of buntanetap and rewritten to cover the new crystal form. Every patent family is now represented for both forms of the Company's drug candidate, securing comprehensive protection, which extends to 2046.
Building on its foundational patent for the treatment of chronic neurodegenerative conditions, the Company has systematically expanded its coverage to include patents for the use of the original compound. Since then, Annovis has developed a robust IP portfolio which includes patents protecting the composition of matter, mechanism of action, applications of buntanetap for multiple indications, and its combination with other drugs.
Recently, the Company identified and characterized a new crystalline form of buntanetap with improved solid-state stability and comparable pharmacokinetic (PK) properties. A series of animal and human studies demonstrated the bioequivalence of both forms of buntanetap as they exhibit identical systemic exposure. These findings were presented at the Alzheimer's Association International Conference (AAIC) 2025 in Toronto, and the poster is now available in the Media Library on the Company's website.
Annovis previously secured protection for the composition of matter and manufacturing process of the crystalline form. However, as of this month, the Company has successfully completed the transfer of all IP inventions, extensions, and combinations from the original buntanetap to the new form, ensuring comprehensive coverage of its drug candidate on two fronts: one preserving the legacy of the semi-crystalline buntanetap (pink) and the other dedicated to the crystalline form (blue).
"This is a major milestone for our company," said Maria Maccecchini, Ph.D., President and CEO of Annovis. "With full IP protection now in place for both forms of buntanetap, we are well-positioned to continue its development and fully explore its therapeutic potential. Buntanetap's unique mechanism of action allows it to suppress multiple pathologies simultaneously, and it may offer even better advantages when complemented by other drugs such as GLP-1 agonists, PDE5 inhibitors, and statins. This opens doors to possible pipeline expansions and broader clinical applications as part of our strategic growth."
This IP milestone for crystal buntanetap complements another significant step – last year the FDA approved the use of the new form for the pivotal Phase 3 clinical trial in early AD (NCT06709014), based on its safety and PK data. The trial was initiated earlier this year with participants (MMSE 21-28, pTau217+) receiving either placebo or crystal buntanetap daily for 18 months. The study is actively underway with over 75 sites secured across the US and more patients entering each week, bringing us closer to reaching our enrollment goal by day. To find more information about the study, visit our updated Patient Portal or the official ClinicalTrials.gov page.
About Annovis
Headquartered in Malvern, Pennsylvania, Annovis is dedicated to addressing neurodegeneration in diseases such as AD and PD. The Company is committed to developing innovative therapies that improve patient outcomes and quality of life. For more information, visit www.annovisbio.com and follow us on LinkedIn, YouTube, and X.
Investor Alerts
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by registering for email alerts at https://www.annovisbio.com/email-alerts.
Forward-Looking Statements
This press release contains forward-looking statements under the Securities Act of 1933 and the Securities Exchange Act of 1934, as amended. Actual results may differ due to various risks and uncertainties, including those outlined in the Company's SEC filings under "Risk Factors" in its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. The Company undertakes no obligation to update forward-looking statements except as required by law.
Contact Information:
Annovis Bio Inc.
101 Lindenwood Drive
Suite 225
Malvern, PA 19355
www.annovisbio.com
Investor Contact:
Alexander Morin, Ph.D.
Director, Strategic Communications
Annovis Bio
[email protected]
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/8d5f14c9-b644-481d-a960-e5b4ad8d1a75


