Aptar's Bidose Nasal System Delivers CARDAMYST™ (etripamil), the First and Only Self-Administered FDA-Approved Nasal Spray for Paroxysmal Supraventricular Tachycardia (PSVT)
Novel treatment for PSVT developed with Milestone Pharmaceuticals
Aptar Group, Inc. (NYSE:ATR), a global leader in drug and consumer product dosing, dispensing and protection technologies, today announced that its Bidose (BDS) Liquid Nasal Spray System is the mechanism for delivering the newly approved CARDAMYST™ (etripamil) Nasal Spray. CARDAMYST received approval by the U.S. Food and Drug Administration (FDA) for the conversion of acute symptomatic episodes of paroxysmal supraventricular tachycardia (PSVT) to sinus rhythm in adults. The novel treatment was developed by Milestone® Pharmaceuticals Inc. (NASDAQ:MIST), a biopharmaceutical company focused on the development and commercialization of innovative cardiovascular medicines. This marks Aptar's first combination of dual Bidose delivery systems housed in a consumer-friendly protective two-pack container.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20251217190273/en/
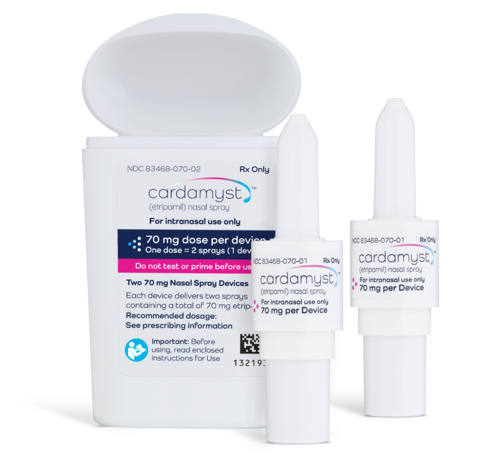
Milestone Pharmaceuticals received U.S. Food and Drug Administration approval of CARDAMYST for the conversion of acute symptomatic episodes of paroxysmal supraventricular tachycardia (PSVT), commonly called SVT, to sinus rhythm in adults.
An estimated two million people in the United States are currently diagnosed with PSVT, a type of arrhythmia or abnormal heart rhythm. PSVT is characterized by episodes of sudden onset rapid heartbeats often exceeding 150 to 200 beats per minute, resulting in an estimated 140,000 to 525,000 Emergency Department visits and 40,000 to 120,000 inpatient hospitalizations in the U.S. each year1.
Integrated Bidose Device Container Closure System Ensures Reliable Drug Access CARDAMYST is easy to use and is small enough to be conveniently carried by patients for use as directed. Aptar CSP Technologies collaborated with Milestone Pharmaceuticals to develop a custom-designed, patient-friendly polypropylene container closure system with a fully integrated cap for CARDAMYST. This protective dual-container system is designed to securely house two Bidose delivery mechanisms, with features intended to prevent accidental activation or dropping from the container and help promote reliability at the moment of need.
Gael Touya, President of Aptar Pharma, said, "This approval underscores the broadening of Aptar's drug delivery solutions for more therapeutic areas and the growing demand for nasal drug delivery. We are pleased that our trusted and proven Bidose nasal system, combined with our protective dual-container system, is now available for patients in yet another therapeutic area."
Alex Theodorakis, President, Aptar Pharma Prescription, added, "After years of close collaboration with Milestone, this successful outcome proves again our capability to support customers globally to develop and launch complex treatments with easy-to-use and reliable systems."
"Aptar has been an essential partner in developing and delivering a novel treatment for PSVT that fills a serious unmet need. We are proud to partner with a company that shares our commitment to innovation and quality and look forward to working together to provide CARDAMYST to patients," said Joseph Oliveto, President and CEO, Milestone Pharmaceuticals.
Proven Nasal Drug Delivery Platforms
Aptar's Bidose (BDS) and Unidose (UDS) systems are designed for intuitive, reliable intranasal administration and for meeting strict U.S. FDA quality standards. Offering effective and proven single or two-shot intranasal delivery for a variety of medicines, these platforms support pharmaceutical partners in developing emergency use treatments.
Accelerated Development Support via Aptar Pharma Services
This innovative treatment for PSVT is another example of a Combination Product submission, and benefited from Aptar Pharma's Drug Services offering, a comprehensive portfolio of stage-specific development packages. Aptar's dedicated Regulatory Affairs experts and analytical scientists help customers proactively address regulatory needs to help expedite approval.
About Aptar
Aptar is a global leader in drug and consumer product dosing, dispensing and protection technologies. Aptar serves a number of attractive end markets including pharmaceutical, beauty, food, beverage, personal care and home care. Using market expertise, proprietary design, engineering and science to create innovative solutions for many of the world's leading brands, Aptar in turn makes a meaningful difference in the lives, looks, health and homes of millions of patients and consumers around the world. Aptar is headquartered in Crystal Lake, Illinois and has over 13,000 dedicated employees in 20 countries. For more information, visit www.aptar.com.
About Milestone Pharmaceuticals
Milestone Pharmaceuticals Inc. (NASDAQ:MIST) is a biopharmaceutical company developing and commercializing innovative cardiovascular medicines to benefit people living with certain heart conditions. Milestone's lead product is CARDAMYST™ (etripamil) nasal spray, a novel calcium channel blocker, which is FDA approved for the conversion of acute symptomatic episodes of paroxysmal supraventricular tachycardia (PSVT) to sinus rhythm in adults. Etripamil is also in development for the treatment of symptomatic episodic attacks associated with atrial fibrillation with rapid ventricular rate, or AFib-RVR. www.milestonepharma.com
This press release contains forward-looking statements, including regarding the anticipated benefits and performance of Aptar's Bidose Nasal System in delivering CARDAMYST, expected patient use and adoption, future market opportunities, and our plans with respect to expanding nasal drug delivery solutions into additional therapeutic areas. Forward-looking statements generally can be identified by the fact that they do not relate strictly to historical or current facts and by use of words such as "expects," "anticipates," "believes," "estimates," "future," "potential," "continues" and other similar expressions or future or conditional verbs such as "will," "should," "would" and "could" are intended to identify such forward-looking statements. Forward-looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results or other events may differ materially from those expressed or implied in such forward-looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment including, but not limited to: the successful commercialization and market acceptance of CARDAMYST; our ability to support customers in the development and launch of drug-device combination products; regulatory requirements and compliance; and competition, including technological advances. For additional information on these and other risks and uncertainties, please see our filings with the Securities and Exchange Commission, including the discussion under "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Form 10-K and Form 10-Qs. We undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
1 PSVT - Areas of Focus - Milestone Pharma
View source version on businesswire.com: https://www.businesswire.com/news/home/20251217190273/en/
Aptar Investors Relations Contact:
Mary Skafidas
[email protected]
+1 347 351 6407
Aptar Pharma Media Contact:
Ciara Jackson
[email protected]
+49 151 1951 6502
Aptar Media Contact:
Katie Reardon
[email protected]
+1 815 479 5671
