Apyx Medical Corporation Receives FDA Clearance for the AYON Body Contouring System™
The AYON Body Contouring System is the first FDA cleared all-in-one platform for the aesthetic surgical suite
Plan to initiate the commercial launch of the AYON Body Contouring System to key opinion leader surgeons in critical geographies during the second half of 2025
CLEARWATER, Fla., May 13, 2025 (GLOBE NEWSWIRE) -- Apyx Medical Corporation (NASDAQ:APYX) ("Apyx Medical"; the "Company"), the manufacturer of a proprietary helium plasma and radiofrequency technology marketed and sold as Renuvion®, is pleased to announce it has received 510(k) clearance from the U.S. Food and Drug Administration (the "FDA") for the AYON Body Contouring System™ ("AYON"). The Company is actively preparing for a commercial launch of AYON with key surgeons in critical geographies starting in the second half of 2025.
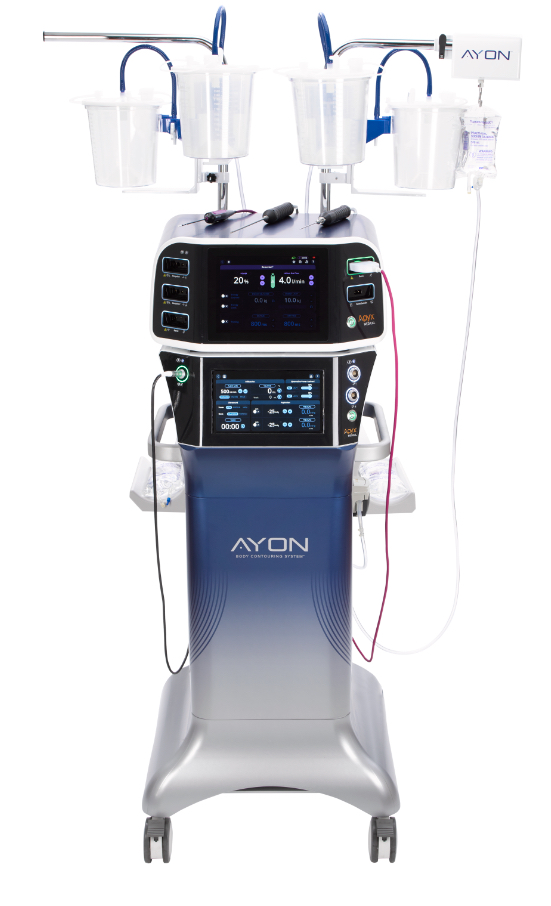
AYON is a groundbreaking, surgeon-designed body contouring system that combines precision, versatility, and innovation in an all-in-one platform. It seamlessly integrates fat removal, closed loop contouring, tissue contraction, and electrosurgical capabilities, empowering surgeons to deliver the most comprehensive body contouring treatments for patients. With advanced features like LIFT Technology for real-time adjustments and Renuvion for enhanced tissue contraction, AYON sets a new standard in surgical care, streamlining procedures and maximizing patient outcomes. Backed by Apyx Medical's expertise and evidence-based design, AYON delivers consistent, reliable performance and an unmatched return on investment. As the first of its kind, AYON is revolutionizing body contouring and shaping the future of aesthetic surgery.
This initial 510(k) clearance for AYON covers a wide variety of aesthetic treatments, including Renuvion to address loose and lax skin, ultrasound-assisted liposuction, electrocoagulation to support procedures requiring removal of excess tissue, along with several others. The Company plans to expand the cleared indications for AYON, to include power liposuction, with an additional 510(k) submission later this year.
"Receiving FDA clearance for the AYON Body Contouring System, which includes our proprietary Renuvion technology for the treatment of loose and lax skin, marks a major step forward in our mission to empower surgeons with transformative tools. By uniting multiple technologies into one powerful platform, AYON is designed to streamline procedures, elevate surgical precision, and ultimately deliver better outcomes for patients," said Charlie Goodwin, President, and CEO of Apyx Medical Corporation. "The aesthetics market is expecting a rapid increase in body contouring procedures over the course of the next year, as the more than 15 million patients currently using GLP-1 drugs achieve rapid weight loss and seek solutions for addressing their loose and lax skin and reshaping their new bodies. AYON provides surgeons with all the necessary solutions in one device for meeting the needs of these patients."
About Apyx Medical Corporation:
Apyx Medical Corporation is an advanced energy technology company with a passion for elevating people's lives through innovative products, including its Helium Plasma Platform Technology products marketed and sold as Renuvion® and now the AYON™ Body Contouring System in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion and J-Plasma offer surgeons a unique ability to provide controlled heat to tissue to achieve their desired results. The effectiveness of Renuvion and J-Plasma are supported by more than 90 clinical documents. The Company also leverages its deep expertise and decades of experience in unique waveforms through OEM agreements with other medical device manufacturers. For further information about the Company and its products, please refer to the Apyx Medical Corporation website at www.ApyxMedical.com.
Cautionary Statement on Forward-Looking Statements:
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including but not limited to, projections of net revenue, margins, expenses, net earnings, net earnings per share, or other financial items; projections or assumptions concerning the possible receipt by the Company of any regulatory approvals from any government agency or instrumentality including but not limited to the U.S. Food and Drug Administration (the "FDA"), supply chain disruptions, component shortages, manufacturing disruptions or logistics challenges; or macroeconomic or geopolitical matters and the impact of those matters on the Company's financial performance.
Forward-looking statements and information are subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company's ability to control or predict. Important factors that may cause the Company's actual results to differ materially and that could impact the Company and the statements contained in this release include but are not limited to risks, uncertainties and assumptions relating to the regulatory environment in which the Company is subject to, including the Company's ability to gain requisite approvals for its products from the FDA and other governmental and regulatory bodies, both domestically and internationally; sudden or extreme volatility in commodity prices and availability, including supply chain disruptions; changes in general economic, business or demographic conditions or trends; changes in and effects of the geopolitical environment; liabilities and costs which the Company may incur from pending or threatened litigations, claims, disputes or investigations; and other risks that are described in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and the Company's other filings with the Securities and Exchange Commission. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Investor Relations Contact:
Jeremy Feffer, Managing Director LifeSci Advisors
OP: 212-915-2568
[email protected]
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c834876c-91f8-482c-b103-bcd18cd8db06


