atai Life Sciences and Beckley Psytech Report Positive Phase 2a Data Demonstrating Improved Outcomes with a Two-Dose Induction Regimen of BPL-003 in Patients with Treatment-Resistant Depression
- Open-label study evaluated a two-dose induction regimen of BPL-003 (8 mg followed by 12 mg two weeks later) in patients with treatment-resistant depression and demonstrated rapid, clinically meaningful and durable antidepressant effects, which are sustained for up to 3 months
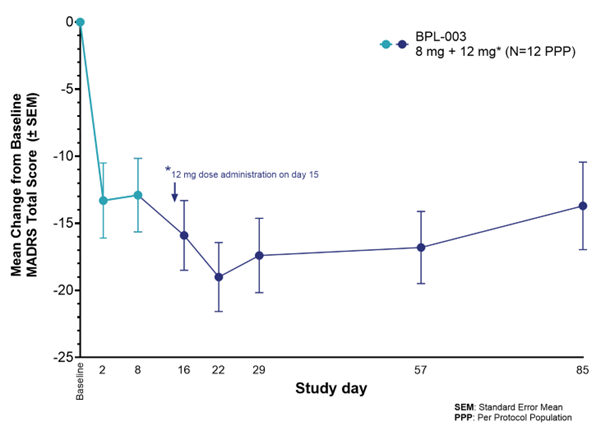
- The second dose of BPL-003 led to further reductions in MADRS scores from baseline, indicating that this regimen has the potential to enhance the clinical response beyond what is observed with a single administration
- Findings will be used alongside data from Beckley Psytech's Phase 2b study of BPL-003 and the soon to be reported Phase 2b open-label extension study to inform the Phase 3 clinical program
NEW YORK and AMSTERDAM and OXFORD, United Kingdom, Sept. 23, 2025 (GLOBE NEWSWIRE) -- atai Life Sciences (NASDAQ:ATAI) ("atai"), a clinical-stage biopharmaceutical company on a mission to develop highly effective mental health treatments to transform patient outcomes, and Beckley Psytech Limited ("Beckley Psytech"), a private clinical-stage biopharmaceutical company pioneering the next generation of mental health treatments, today announced positive data from a proof-of-concept study investigating a two-dose induction regimen of BPL-003 (intranasal mebufotenin benzoate), in patients with treatment-resistant depression (TRD).
The open-label Phase 2a study (NCT05660642) enrolled 13 patients with TRD who were not on concurrent antidepressants, and 12 met the criteria for per-protocol analysis. Patients were given an 8 mg dose of BPL-003 followed by a 12 mg dose two weeks later. Safety, tolerability and efficacy assessments were conducted at various timepoints for 12 weeks following the initial dose using multiple validated depression rating scales including the MADRS (Montgomery-Asberg Depression Rating Scale). The results demonstrate that a second dose of BPL-003 at Week 2 has the potential to induce greater antidepressant effects, as evidenced by additional reductions in MADRS scores, without impact on the safety and tolerability profile of the treatment.
Key Findings:
- Rapid and durable efficacy: Following the first (8 mg) dose, patients experienced a mean MADRS reduction of 13.3 points from baseline at Day 2 and a mean MADRS reduction of 12.9 at Day 8. One week after the second dose (12 mg), there was a further decrease in MADRS score to a total of a 19.0-point reduction from baseline, with sustained antidepressant effects observed through Week 12 (13.7 points from baseline). These findings suggest that a second dose of BPL-003 may further enhance the clinical response beyond what is achieved with a single administration.
- Improved response and remission rates: The second dose of BPL-003 increased the proportion of patients meeting response and remission criteria for depression. Remitter rates one week after the initial 8mg dose were 25%, with rates doubling to 50% at Week 8 (6 weeks after the second dose administration) and 42% at Week 12.
- Strong and consistent tolerability profile: BPL-003 was shown to be generally well-tolerated, with all adverse events classified as mild to moderate, and there were no severe or serious drug-related adverse events reported. These findings are consistent with those seen in other previously reported studies of BPL-003.
- Practical administration: Patients met discharge readiness criteria within two hours after dosing for both doses, reinforcing the potential for BPL-003 to be integrated into the established interventional psychiatric treatment paradigm.
Cosmo Feilding Mellen, Chief Executive Officer and Co-Founder of Beckley Psytech, said: "These results underline BPL-003's potential to offer a rapid, well-tolerated and durable treatment option for patients with treatment-resistant depression. They suggest that a second dose of BPL-003 not only maintains, but potentially deepens, antidepressant effects while remaining well-tolerated. Importantly, the findings are consistent with results from earlier Phase 2a cohorts, including studies in patients who were taking antidepressants (SSRIs), where a single dose of BPL-003 produced rapid and sustained improvements for up to three months. Together with the results from our Phase 2b program, these data provide a strong foundation of evidence to design our Phase 3 program, and we are grateful to the patients, investigators and sites who have made this progress possible."
The results from this study add to the growing body of evidence from the BPL-003 clinical program demonstrating that BPL-003 has the potential to be a rapid-acting, durable and convenient treatment option for patients with TRD. Doses for the study are the same as the active doses used in the core, blinded stage of Beckley Psytech's Phase 2b study of BPL-003, which reported positive topline findings in July. Those results showed that a single 8 mg or 12 mg dose produced statistically significant and clinically meaningful antidepressant effects at Day 2, Day 8, Day 29 and Day 57 after dosing, compared with a 0.3 mg comparator dose.
Dosing and follow-up in the open-label extension (OLE) stage of the Phase 2b study, which is evaluating the effects of a 12 mg dose of BPL-003 administered eight weeks after the initial dose in the core study, is complete and data are expected in October.
atai and Beckley Psytech are finalizing plans to engage with the U.S. Food and Drug Administration (FDA) and other regulatory agencies to discuss the design of the Phase 3 clinical trials for BPL-003 in patients with TRD. Pending FDA feedback, initiation of the Phase 3 clinical program is expected in the first half of 2026.
Srinivas Rao, Chief Executive Officer and Co-Founder of atai Life Sciences, said: "This proof-of-concept study provides compelling evidence that a two-dose induction regimen for BPL-003 may optimize outcomes for patients with treatment-resistant depression. This dosing approach could be further explored in Phase 3 studies, subject to discussions with regulators."
About BPL-003
BPL-003 is Beckley Psytech's patent-protected, proprietary intranasal formulation of mebufotenin benzoate, administered via a nasal spray device used in a previously approved drug product. BPL-003 is designed to deliver rapid and durable effects from a single dose, with a short treatment window, and is being investigated as a potential therapy for treatment-resistant depression (TRD) and for alcohol use disorder (AUD). BPL-003 is covered by granted US, UK and European composition-of-matter patents, with multiple further claims pending in various jurisdictions.
About treatment-resistant depression
Depression is a debilitating and life-changing condition affecting nearly 300 million people across the globe, with around 52 million people affected by the condition in Europe and the US combined. Treatment-resistant depression occurs when an individual does not respond to two or more courses of antidepressants and some studies show that it may affect up to 50% of those living with depression, meaning there is a significant unmet need for more effective treatments.
About atai Life Sciences
atai is a clinical-stage biopharmaceutical company on a mission to develop highly effective mental health treatments to transform patient outcomes. atai's pipeline of psychedelic-based therapies includes VLS-01 (buccal film DMT) for treatment-resistant depression (TRD) and EMP-01 (oral R-MDMA) for social anxiety disorder, which are in Phase 2 clinical development. It is also advancing a drug discovery program to identify novel, non-hallucinogenic 5-HT2AR agonists for TRD. These programs aim to address the complex nature of mental health by providing commercially scalable interventional psychiatry therapies that can integrate seamlessly into healthcare systems. In June 2025, it was announced that atai Life Sciences and Beckley Psytech would be combining to create a global leader in psychedelic mental health therapies. The all-share transaction is expected to close in the second half of 2025. For the latest updates and to learn more about atai's mission, visit www.atai.com or follow the Company on LinkedIn and on X.
About Beckley Psytech
Beckley Psytech Ltd is a private biopharmaceutical company dedicated to improving the lives of people living with neuropsychiatric disorders by developing rapid-acting psychedelic medicines. Founded in 2019, and underpinned by more than two decades of pioneering scientific research from the Beckley Foundation, Beckley Psytech combines world-leading psychedelic science with extensive drug development expertise in order to optimise patient outcomes, improve treatment opportunities and ease the burden these conditions have on individuals, healthcare systems and society. For more information about Beckley Psytech, visit www.beckleypsytech.com or follow the Company on LinkedIn.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). The words "believe," "may," "will," "estimate," "continue," "anticipate," "intend," "expect," "anticipate," "initiate," "could," "would," "project," "plan," "potentially," "preliminary," "likely," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements include express or implied statements relating to, among other things: expectations regarding the closing of the acquisition of Beckley Psytech Limited (the "Proposed Transactions"), including timing and approvals; progress on and results of Beckley Psytech's BPL-003 trials and related data readouts, including Phase 2a data, the expected timing of Phase 2b data, and the timing of regulatory discussions with respect to Phase 3 trial design for BPL-003; and the potential benefits of BPL-003 for patients with TRD.
Forward-looking statements are neither promises nor guarantees, but involve known and unknown risks and uncertainties that could cause actual results to differ materially from those projected, including, without limitation, (i) the Proposed Transaction may not be completed in a timely manner or at all, including the risk that any required shareholder approvals are not obtained; (ii) the failure to realize the anticipated benefits of the Proposed Transaction; (iii) the possibility that any or all of the various conditions to the consummation of the Proposed Transaction may not be satisfied or waived; (iv) the occurrence of any event, change or other circumstance that could give rise to the termination of the share purchase agreement; and (v) the effect of the announcement or pendency of the Proposed Transaction on atai's ability to retain and hire key personnel, or its operating results and business generally and other important factors described in the section titled "Risk Factors" in our most recent Annual Report on Form 10-K filed with the SEC and the registration statement on Form S-4 that was filed with the SEC on September 22, 2025 (the "Registration Statement"), as such factors may be updated from time to time in atai's other filings with the SEC. atai disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by applicable law.
No Offer or Solicitation
This press release is for information purposes only and is not intended to and does not constitute, or form part of, an offer, invitation or the solicitation of an offer or invitation to purchase, otherwise acquire, subscribe for, sell or otherwise dispose of any securities, or the solicitation of any vote or approval in any jurisdiction, pursuant to the Proposed Transactions or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law.
Additional Information and Where to Find It
This press release is being made in respect of the Proposed Transactions. In connection with the Proposed Transactions, the Registration Statement was filed with the SEC on September 22, 2025 and included a proxy statement of the Company (the "Proxy Statement"), as well as other relevant documents regarding the Proposed Transactions. This communication is not a substitute for the Registration Statement, the Proxy Statement or any other document which the Company has or may file with the SEC. INVESTORS ARE URGED TO READ IN THEIR ENTIRETY THE REGISTRATION STATEMENT, INCLUDING THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTIONS, AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY CONTAIN IMPORTANT INFORMATION.
A free copy of the Registration Statement, including the Proxy Statement, as well as other filings containing information about the Company, may be obtained at the SEC's website (http://www.sec.gov).
Participants in the Solicitation
The Company and its directors and executive officers may be deemed to be participants in the solicitation of proxies from its shareholders in respect of the proposed transactions contemplated by the Registration Statement, including the Proxy Statement. Information regarding the persons who are, under the rules of the SEC, participants in the solicitation of the shareholders of the Company in connection with the proposed transactions, including a description of their direct or indirect interests, by security holdings or otherwise, is set forth in the Registration Statement that was filed with the SEC on September 22, 2025, including the Proxy Statement. Information regarding the Company's directors and executive officers is also contained in its Annual Report on Form 10-K for the year ended December 31, 2024 and its proxy statement on Schedule 14A, dated April 21, 2025, which are filed with the SEC.
Contact Information
Investor Contact:
[email protected]
atai Media Contact:
[email protected]
Beckley Psytech Media Contact:
[email protected]
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/51b5d843-f2ae-4ef0-bce3-901e786bb39d


