- Repeated positive recommendation from Data Safety Monitoring Board ("DSMB") of pivotal Phase 3 study for lead clinical candidate Bria-IMT™ in combination with checkpoint inhibitor
- Bria-IMT has received Fast Track designation from FDA and patient enrollment has been accelerating in the pivotal Phase 3 study in metastatic breast cancer
- Successful completion of the pivotal study may lead to a Biologics License Application submission, Priority Review, Full Approval, and commercialization
- Interim analysis planned after 144 events (deaths) in Phase 3 Study
- Phase 2 survival data superior to reported standard of care including TRODELVY® (sacituzumab govitecan-hziy) in similar hormone receptor positive (HR+) metastatic breast cancer patients
- Promising early results for OTS platform technology with resolution of breast cancer metastasis in first patient dosed
PHILADELPHIA and VANCOUVER, British Columbia, May 20, 2025 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (NASDAQ:BCTX, BCTXW, BCTXZ)), (TSX:BCT) ("BriaCell" or the "Company"), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to issue a letter to shareholders from Dr. William V. Williams, BriaCell's President and CEO.
Dear BriaCell Shareholders,
I am writing this letter to share our excitement regarding the recent milestones achieved in our pivotal Bria-IMT™ Phase 3 study ("Phase 3 study"), impressive survival benefit updates from our Bria-IMT Phase 2 study ("Phase 2 study"), and highly encouraging early data of our newest Bria-OTS™ Phase 1/2 personalized cancer treatment study. We firmly believe that our novel immunotherapy candidates have the potential to transform the treatment landscape for cancer patients.
Bria-IMT Pivotal Phase 3 Clinical Study:
Bria-IMT™, an off-the-shelf targeted cell-based immunotherapy for the treatment of metastatic breast cancer, is a genetically engineered human breast cancer cell line designed to stimulate the immune system to attack cancer. Bria-IMT is being investigated in combination with an immune checkpoint inhibitor to enhance its therapeutic efficacy in a Phase 3 study.
Recently, we announced that over 75 patients have been enrolled at 54 active clinical sites across 15 states. These include large and well-known sites such as the Sylvester Comprehensive Cancer Center of the University of Miami (an NCI-designated Cancer Center), the Robert H. Lurie Comprehensive Cancer Center of Northwestern University (an NCI-designated Cancer Center), and Texas Oncology-Baylor Charles A. Sammons Cancer Center. We have repeatedly received positive recommendations from our DSMB and are progressing towards completion of the study. Successful completion of the pivotal study would allow us to submit an application for full drug approval under our fast-track designation which would greatly accelerate the path to commercialization.
At the 2025 American Association for Cancer Research("AACR") which took place in Chicago, IL from April 25th to April 30th we presented our Late-Breaker clinical data highlighting a positive tolerability profile and identification of potential response biomarkers.
We will have a poster presentation updating the Phase 3 study at the 2025 American Society of Clinical Oncology ("ASCO") Annual Meeting which is taking place from May 30th to June 3rd at McCormick Place, Chicago, IL.
Bria-IMT Phase 2 Clinical Study:
We continue to follow patients' survival and clinical data from the Phase 2 portion of the study. We recently reported Phase 2 survival data superior to reported standard of care including TRODELVY® (sacituzumab govitecan-hziy) in similar hormone receptor positive (HR+) metastatic breast cancer patients. Clinical benefit was observed in 83% of evaluable patient sub-group who were treated with our Phase 3 formulation, which we believe will translate to favorable outcomes for our ongoing Phase 3 patients.
These women are in desperate need of therapy to extend their lives. The average survival in this very sick patient population is under one year and can be as little as few weeks for women who have progressed through multiple regimens.
Bria-OTS Phase 1/2 Clinical Study
BriaCell Personalized Off-The-Shelf Immunotherapy Platform
Bria-OTS or Off-The-Shelf is our proprietary approach designed to maximize patient response by matching each patient's human leukocyte antigen ("HLA") to provide personalized immunotherapy. Utilizing a simple saliva test, we identify HLA and then treat patients with premade HLA matched cells. This novel treatment was developed upon our discovery that HLA matched Bria-IMT cell-lines generated the best clinical response in our Phase 1/2a Bria-IMT study.
We recently confirmed 100% resolution of a lung metastasis (Figure 1) with Bria-OTS monotherapy (single agent) at 4 month follow-up in a hormone receptor positive ("HR+") breast cancer patient who was first noted to have tumor resolution at 2 months. This patient remains on study with stable disease elsewhere. This is a remarkable response in such a short period of time.
Figure 1: Treatment with Bria-OTS monotherapy resulted in 100% resolution of a breast cancer tumor in the lung of the metastatic breast cancer (MBC) patient following 2 months of therapy and confirmed at 4 months of therapy (axial and coronal views)
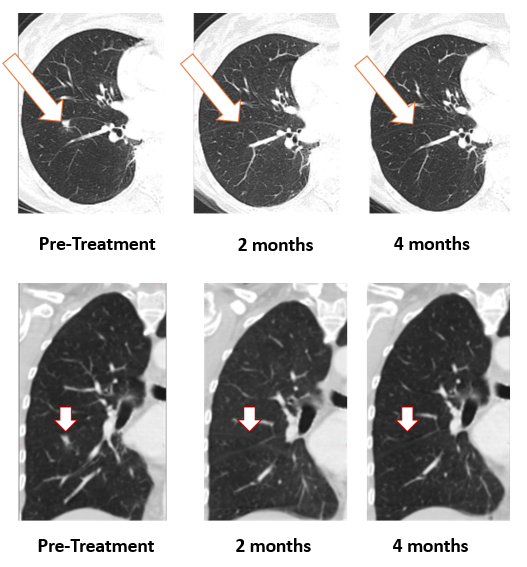
The Phase 1/2a clinical trial in metastatic breast cancer is a dose escalation study initially evaluating the safety and efficacy of Bria-OTS as monotherapy and will be followed by Bria-OTS in combination with an immune checkpoint inhibitor.
Financing
We recently raised US$13.8 million, through an underwritten public offering, that will be used to support our clinical programs, working capital requirements, general corporate purposes, and the advancement of our business.
We continue to strive towards solutions for cancer patients whose medical needs are unmet and look forward to sharing more exciting news with you in the coming months.
I would like to thank all our stakeholders for their continued support – shareholders, employees, board members, medical advisory board, scientific advisory board, investigators, and clinical teams who have worked tirelessly to make these clinical advancements possible. Finally, I would like to offer our sincerest thanks to our patients and their families for their patience and trust in our science and technology. I am looking forward to sharing significant program advancements in the coming months.
Thank You!
Yours very truly,
William V. Williams, MD
President & CEO
BriaCell Therapeutics Corp.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include statements regarding whether successful completion of the pivotal study may lead to a Biologics License Application submission, Priority Review, Full Approval, and commercialization; whether BriaCell's novel immunotherapy candidates have the potential to transform the treatment landscape for cancer patients; whether BriaCell will be able to share significant program advancements in the coming months; whether successful completion of the pivotal study would allow BriaCell to submit an application for full drug approval under its fast-track designation and whether such designation would greatly accelerate the path to commercialization; BriaCell presenting a poster updating the Phase 3 study at ASCO; BriaCell's belief that observed clinical benefit in 83% of evaluable patient sub-group who were treated with its Phase 3 formulation will translate to favorable outcomes for BriaCell's ongoing Phase 3 patients; and whether the Phase 1/2a clinical trial in metastatic breast cancer (a dose escalation study initially evaluating the safety and efficacy of Bria-OTS as monotherapy) will be followed by Bria-OTS in combination with an immune checkpoint inhibitor. These statements are based on BriaCell's current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading "Risks and Uncertainties" in the Company's most recent Management's Discussion and Analysis, under the heading "Risk Factors" in the Company's most recent Annual Information Form and under "Risks and Uncertainties" in the Company's other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
[email protected]
Investor Relations Contact:
[email protected]
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/fe251557-2c60-4a30-87d4-03018fc8b225


