BriaCell Quadruples Progression Free Survival (PFS) in Patient with "Eye-Bulging" Metastatic Breast Cancer
- Progression free survival (PFS) extended to 9.1 months in ADC resistant patient - quadruple the PFS of patients in similar studies 1, 2, 3
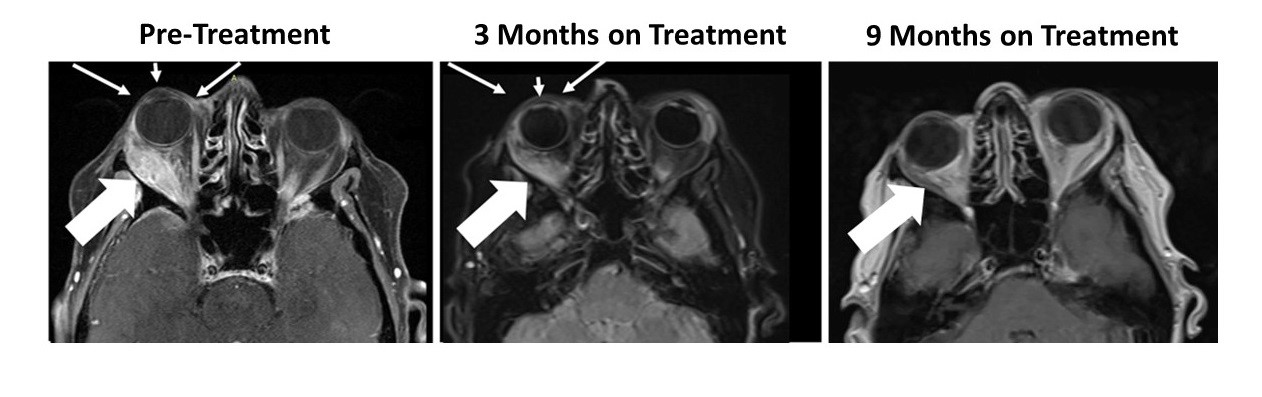
- Significant reduction of "Eye-Bulging" metastatic breast cancer tumor was previously reported
- Heavily pre-treated patient had failed 8 prior regimens including antibody-drug conjugate (ADC) therapy and continues to receive BriaCell treatment
PHILADELPHIA and VANCOUVER, British Columbia, July 18, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (NASDAQ:BCTX, BCTXW))) (TSX:BCT) ("BriaCell" or the "Company"), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to report significantly higher PFS for its top responder patient in the Phase 2 study of BriaCell's Bria-IMT™ regimen in combination with an immune checkpoint inhibitor in metastatic breast cancer. The patient remains alive and she continues to receive BriaCell's treatment regimen.
"We are extremely pleased with the unprecedented survival benefit in this very-difficult-to-treat patient," stated Dr. William V. Williams, BriaCell's President and CEO. "This data represents a step forward in our efforts to build on our knowledge and successes to transform cancer care for patients. We expect to replicate this positive data in our ongoing Phase 3 study and bring relief to cancer patients whose medical needs remain unmet."
"Despite recent advances in cancer therapy, metastatic breast cancer remains an unmet medical need, as current treatments are limited by poor survival and harsh side effects," commented Dr. Giuseppe Del Priore, BriaCell's Chief Medical Officer. "The Bria-IMT™ regimen produced a much longer than expected survival benefit in addition to its favorable safety and tolerability in this patient suggesting its potential as a therapeutic option for these cancer patients."
The patient had a large right orbital lesion (behind the right eye) and a right temporal lobe lesion (in the right side of the brain). The temporal lobe lesion is no longer detectable, while the orbital lesion has continued to shrink markedly (see figure showing resolution of proptosis post treatment (small arrows) with reduction in tumor indicated by the large arrows). In addition, her tumor markers (blood tests that correlate with the amount of tumor in the body) have markedly decreased from her pre-treatment levels.
Figure 1: Responder Images - Bria-IMT™ Regimen
| Table 1: Bria-IMT™ and historical clinical data in MBC patients who failed multiple prior treatments | |
| Study | PFS (months) |
| Top Responder Patient | 9.1+ |
| Bardia, A. et. al. 1 | 1.7 |
| Tripathy D. et. al. 2 | 1.9 |
| O'Shaughnessy J. et. al. non-TNBC 3 | 2.3 |
| O'Shaughnessy J. et. al. TNBC 3 | 1.6 |
1,2,3 Data is shown for the intent to treat population for the control group treated with treatment of physician's choice, which is the comparator in the BriaCell's ongoing pivotal Phase 3 study.
2 This paper describes patients with brain metastases, which were also present in the patient described.
+ Indicates the patient is ongoing in the study.
References
- Bardia A, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024 May 20;42(15):1738-1744. doi: 10.1200/JCO.23.01409. Epub 2024 Feb 29. PMID: 38422473.
- Tripathy D, et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases: final results from the phase 3 ATTAIN randomized clinical trial. JAMA Oncol. 2022;8(7):1047-1052. doi:10.1001/jamaoncol.2022.0514.
- O'Shaughnessy J et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022 Sep;195(2):127-139. doi: 10.1007/s10549-022-06602-7. Epub 2022 May 11. PMID: 35545724; PMCID: PMC9374646.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about BriaCell replicating positive data in its ongoing Phase 3 study; BriaCell's Bria-IMT™ regimen bringing relief to cancer patients whose medical needs remain unmet; and the Bria-IMT™ regimen becoming a therapeutic option for metastatic breast cancer patients, are based on BriaCell's current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading "Risks and Uncertainties" in the Company's most recent Management's Discussion and Analysis, under the heading "Risk Factors" in the Company's most recent Annual Information Form, and under "Risks and Uncertainties" in the Company's other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
[email protected]
Media Relations:
Jules Abraham
CORE IR
[email protected]
Investor Relations Contact:
CORE IR
[email protected]
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/cef51e5b-ff25-4c3e-929d-22ad6df22927


