Corcept Presents Results from Phase 2 Study of Dazucorilant in Patients with Amyotrophic Lateral Sclerosis (ALS) at ENCALS 2025 Annual Meeting
- DAZALS did not meet its primary endpoint of improved outcome in the ALS Functional Rating Scale-Revised (ALSFRS-R) in patients who received dazucorilant compared to patients who received placebo
- DAZALS met its secondary endpoint of improved overall survival at week 24 of the study in patients who received 300 mg of dazucorilant compared to patients who received placebo
- Exploratory analysis at the one-year mark shows continued significant improvement in overall survival between patients who received 300 mg of dazucorilant and those who received placebo only
- Corcept seeking guidance from United States and European regulators on optimum path forward
Corcept Therapeutics Incorporated (NASDAQ:CORT), a commercial-stage company engaged in the discovery and development of medications to treat severe endocrinologic, oncologic, metabolic and neurologic disorders by modulating the effects of the hormone cortisol, presented results from its DAZALS study of dazucorilant in patients with ALS at the European Network to Cure ALS (ENCALS) 2025 annual meeting. The presentation can be found here.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250605516942/en/
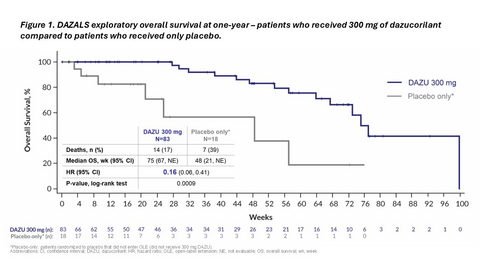
Figure 1. DAZALS exploratory overall survival analysis at one-year – patients who received 300 mg of dazucorilant compared to patients who received only placebo.
DAZALS is a randomized, double-blind, placebo-controlled Phase 2 study in which 249 patients with ALS were randomized to receive either 150 mg of dazucorilant, 300 mg of dazucorilant or placebo, daily for 24 weeks. Patients who completed the treatment period were eligible to enroll in a long-term extension study in which all patients received 300 mg of dazucorilant. The primary endpoint in DAZALS was the difference in ALSFRS-R between patients who received dazucorilant and those who received placebo. Overall survival was a secondary endpoint.
Although DAZALS did not meet its primary endpoint, patient survival significantly improved. At week 24 of the study, no deaths had occurred in the 83 patients who received 300 mg of dazucorilant, while there were five deaths in the 82-patient placebo group (p-value of 0.02).
An exploratory analysis conducted at the one-year mark shows the survival benefit has continued. Patients randomized to 300 mg of dazucorilant lived significantly longer than patients who received placebo and did not switch to 300 mg of dazucorilant in the extension study. The difference between groups was pronounced, with a hazard ratio of 0.16 (p-value: 0.0009). See Figure 1.
A similar survival benefit was observed in patients who received 300 mg of dazucorilant for greater than 24 weeks, either in the treatment period or in the extension study, compared to patients who received either placebo or 150 mg of dazucorilant for 24 weeks and did not receive dazucorilant in the extension study (hazard ratio: 0.36; p-value 0.02). See Figure 2. The extension study is ongoing.
Dazucorilant has demonstrated an acceptable safety profile, with 92 percent of adverse events being mild to moderate in severity. The frequency of severe and serious adverse events in patients who received dazucorilant was similar to those who received placebo. Mild to moderate, dose-related, transient abdominal pain was the most common adverse effect.
"The improvement in overall survival, first noted in the DAZALS study at six months, continues to be seen at one-year. This finding deserves our full attention in service to patients with this tragic disease. Progress in the development of new ALS treatments is of critical importance," said Leonard H. van den Berg, M.D., Ph.D., Professor and Chair in the Department of Neurology, UMC Utrecht Brain Centre, Utrecht, The Netherlands, and Principal Investigator in the DAZALS study.
"Medications that can extend life for patients with ALS are urgently needed. We are working with regulatory authorities to determine the optimal path for advancing dazucorilant," said Bill Guyer, PharmD, Corcept's Chief Development Officer. "We would like to thank the patients, their families and care partners, as well as the investigators, doctors and clinic staff involved in this study."
About the DAZALS Study
DAZALS is a randomized, double-blind, placebo-controlled Phases 2 trial in which 249 patients with ALS were randomized 1:1:1 to receive either 150 mg of dazucorilant, 300 mg of dazucorilant or placebo daily for 24 weeks. Patients who completed the treatment period were eligible to enroll in the long-term extension study in which all patients received 300 mg of dazucorilant. Baseline patient characteristics, including the ENCALS risk score, time from diagnosis, ALSFRS-R total score, and bulbar onset, were consistent across study arms.
The DAZALS primary endpoint was the difference in change from baseline during the study's 24-week treatment period in ALSFRS-R score between patients who received dazucorilant and those who received placebo. Key secondary endpoints include overall survival and quality of life. DAZALS was conducted at sites in Europe, the United States and Canada.
About Amyotrophic Lateral Sclerosis (ALS)
ALS, also known as Lou Gehrig's disease or motor neuron disease, is a fatal degenerative neurologic disorder that affects more than 55,000 people in the United States and Europe. ALS causes muscles to weaken and, as the disease progresses, severely impairs patients' ability to speak, eat, move and breathe. There is increasing evidence that patients with ALS, particularly those with rapid disease progression, exhibit elevated or abnormal cortisol levels. A patient's life expectancy after diagnosis is two to five years.
About Dazucorilant
Dazucorilant is a selective cortisol modulator that binds to the glucocorticoid receptor but does not bind to the body's other hormone receptors. Corcept is studying it as a potential treatment for ALS and other neurologic disorders. Dazucorilant is proprietary to Corcept and is protected by composition of matter, method of use and other patents. The U.S. Food and Drug Administration has granted dazucorilant Fast Track Designation and orphan drug status for the treatment of ALS in the United States.
About Corcept Therapeutics
For over 25 years, Corcept has focused on cortisol modulation and its potential to treat patients with a wide variety of serious disorders and has discovered more than 1,000 proprietary selective cortisol modulators and glucocorticoid receptor antagonists. Corcept is conducting advanced clinical trials in patients with hypercortisolism, solid tumors, ALS and liver disease. In February 2012, the company introduced Korlym®, the first medication approved by the U.S. Food and Drug Administration for the treatment of patients with endogenous hypercortisolism. Corcept is headquartered in Redwood City, California. For more information, visit Corcept.com.
Forward-Looking Statements
Statements in this press release, other than statements of historical fact, are forward-looking statements based on our current plans and expectations, which are subject to risks and uncertainties that might cause our actual results to differ materially from those such statements express or imply. These risks and uncertainties are set forth in our SEC filings, which are available at our website and the SEC's website.
In this press release, forward-looking statements include those concerning the development of dazucorilant as a treatment for patients with ALS, including the pace, conduct, timing and outcome of DAZALS and its associated long-term extension study, as well as oversight or requirements that may be imposed by the FDA or other regulatory authorities. We disclaim any intention or duty to update forward-looking statements made in this press release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250605516942/en/
Investor inquiries:
[email protected]
Media inquiries:
[email protected]
www.corcept.com
