Cytokinetics Presents Additional Data From GALACTIC-HF at the American Heart Association Scientific Sessions 2024
SOUTH SAN FRANCISCO, Calif., Nov. 16, 2024 (GLOBE NEWSWIRE) -- Cytokinetics, Incorporated (NASDAQ:CYTK) today announced new data from post-hoc analyses of GALACTIC-HF (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure), the Phase 3 cardiovascular outcomes clinical trial of omecamtiv mecarbil were presented at the American Heart Association Scientific Sessions 2024 in Chicago, IL.
"These analyses reinforce the potential treatment benefit of omecamtiv mecarbil in patients from GALACTIC-HF who are at higher risk, such as older patients or those with a recent ventricular arrhythmia," said Stuart Kupfer, M.D., Senior Vice President, Chief Medical Officer. "As we prepare for the start of COMET-HF, the confirmatory Phase 3 clinical trial of omecamtiv mecarbil, we look forward to evaluating its potential to reduce the risk of adverse heart failure outcomes across the spectrum of these very high-risk patients failing guideline-directed medical therapy."
Treatment with Omecamtiv Mecarbil Reduced Risk of the Primary Composite Endpoint in Patients with Severe Heart Failure Independent of Age
A post-hoc analysis from GALACTIC-HF supports the potential efficacy of omecamtiv mecarbil irrespective of age, including in patients with severe heart failure. Of the 8,232 patients included in the analysis, patients were on average 64.5 years old, with 54.5% aged ≥65 years. At baseline, patients aged ≥65 years were more likely to be women, have atrial fibrillation or flutter, have worse New York Heart Association (NYHA) Functional Class and have higher NT-proBNP as compared to patients aged <65 years. Over a median follow-up period of 21.8 months, the primary composite endpoint of cardiovascular death or heart failure events occurred at a rate of 21.4 per 100 patient years among patients <65 years, and 28.8 per 100 patient years in patients ≥65 years. In the overall study population, the treatment effect of omecamtiv mecarbil was similar across age groups (interaction p=0.73). In patients with severe heart failure, omecamtiv mecarbil reduced the risk for the primary outcome independent of age group (HR 0.77; 95% CI, 0.64-0.92 and HR 0.83; 95% CI, 0.71-0.97 for patients <65 years and ≥65 years respectively; interaction p=0.72). These relative risk reductions were associated with large absolute risk reductions (8.6 and 7.9, respectively). Additionally, adverse events of special interest, such as ventricular arrhythmias (VA) and myocardial ischemic events were similar between omecamtiv mecarbil and placebo in both age groups.
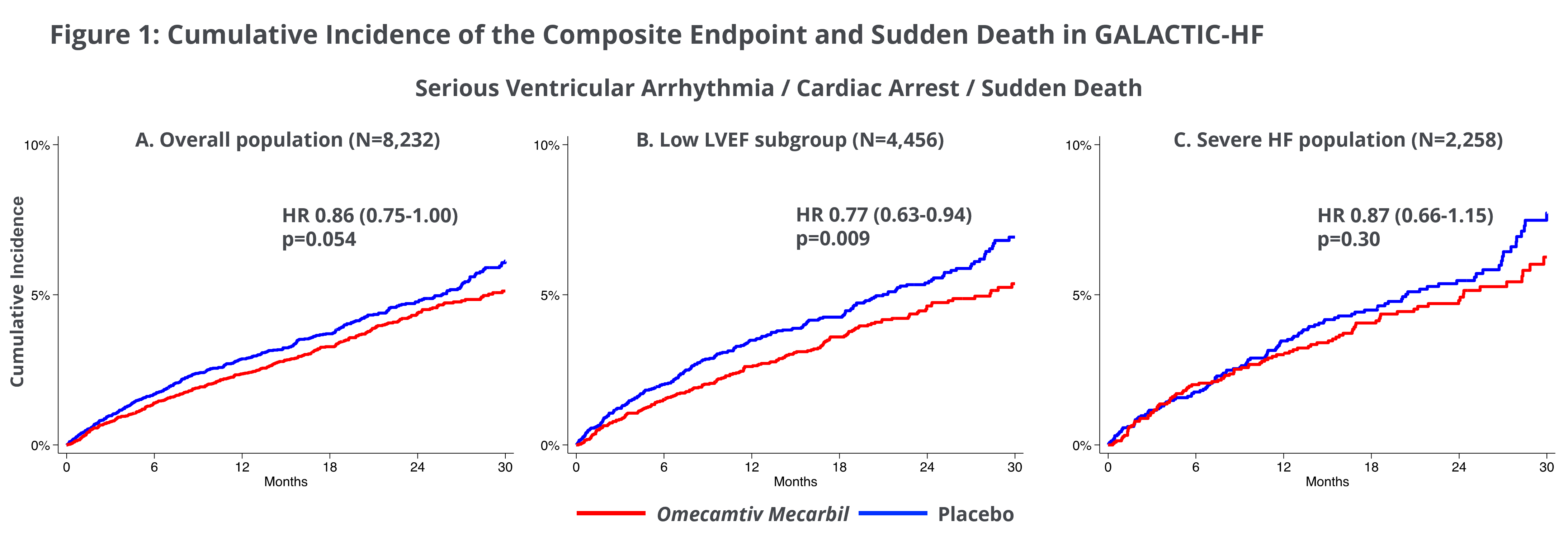
Ventricular Arrythmias Associated with Adverse Clinical Outcomes in Patients with Heart Failure and Reduced Ejection Fraction; Treatment with Omecamtiv Mecarbil Reduces Risk of Ventricular Arrythmias in Patients with Severely Reduced LVEF
A second post-hoc analysis from GALACTIC-HF investigated clinical consequences of ventricular arrhythmias (VA) in the patients studied in GALACTIC-HF. Of the 8,232 patients enrolled in GALACTIC-HF, following an occurrence of VA, there was a significantly higher risk of the primary endpoint of cardiovascular death or heart failure events (HR 1.67; 95% CI 1.42-1.97; p<0.001) and all-cause mortality (HR 2.07; 95% CI 1.77-2.42; p<0.001). Treatment with omecamtiv mecarbil was not associated with an increase in the occurrence of VA and the occurrence of VA in participants treated with omecamtiv mecarbil did not impart worse prognosis than those with VA compared to placebo. Of note, participants with lower LVEF had an increased risk of VA, and in those participants, treatment with omecamtiv mecarbil appeared to reduce the risk for the composite outcome of VA, cardiac arrest and sudden death (LVEF ≤28%, HR 0.77, 95% CI 0.63-0.94; p=0.009, Figure 1). Overall, these findings support the safety of omecamtiv mecarbil with regards to VA as well as a potential benefit in patients with severely reduced LVEF and are consistent with the findings of increased treatment effect of omecamtiv mecarbil in patients with severely reduced LVEF.
About Omecamtiv Mecarbil
Omecamtiv mecarbil is an investigational, selective, small molecule cardiac myosin activator, the first of a novel class of myotropes1 designed to directly target the contractile mechanisms of the heart, binding to and recruiting more cardiac myosin heads to interact with actin during systole. Omecamtiv mecarbil is designed to increase the number of active actin-myosin cross bridges during each cardiac cycle and consequently augment the impaired contractility that is associated with heart failure with reduced ejection fraction (HFrEF). Preclinical research has shown that omecamtiv mecarbil increases cardiac contractility without increasing intracellular myocyte calcium concentrations or myocardial oxygen consumption.2-4
The development program for omecamtiv mecarbil assessed its potential for the treatment of HFrEF. Positive results from GALACTIC-HF, the first Phase 3 clinical trial of omecamtiv mecarbil demonstrated a statistically significant effect of treatment with omecamtiv mecarbil to reduce risk of the primary composite endpoint of cardiovascular (CV) death or heart failure events (heart failure hospitalization and other urgent treatment for heart failure) compared to placebo in patients treated with standard of care. No reduction in the secondary endpoint of time to CV death was observed. In general, the overall rates of myocardial ischemia, ventricular arrhythmias and death were similar between treatment and placebo groups. Adverse events and treatment discontinuation of study drug were balanced between treatment arms. The magnitude of the treatment effect in a pre-specified subgroup of more than 4,000 patients with heart failure with severely reduced ejection fraction (<30%) was observed to be greater than in the overall drug treated population of GALACTIC-HF. Omecamtiv mecarbil will be the subject of COMET-HF (Confirmation of Omecamtiv Mecarbil Efficacy Trial in Heart Failure), a confirmatory Phase 3 clinical trial in patients with symptomatic heart failure with severely reduced ejection fraction, expected to start in Q4 2024.
About Heart Failure with Severely Reduced Ejection Fraction
Heart failure is a grievous condition that affects more than 64 million people worldwide5 about half of whom have reduced left ventricular function.6,7 It is the leading cause of hospitalization and readmission in people age 65 and older.8,9 By 2029 is it estimated that 2.8 million people in the U.S. will have heart failure with severely reduced ejection fraction10, characterized as heart failure with reduced ejection fraction (HFrEF) <30%, and 840,000 people will have severely reduced ejection fraction with other features indicative of high risk heart failure.11 Patients with high risk heart failure with severely reduced ejection fraction account for approximately 60% of all HFrEF hospitalizations, with 35% of patients re-hospitalized within a year. 12,13
About Cytokinetics
Cytokinetics is a late-stage, specialty cardiovascular biopharmaceutical company focused on discovering, developing and commercializing muscle biology-directed drug candidates as potential treatments for debilitating diseases in which cardiac muscle performance is compromised. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact myocardial muscle function and contractility. Following positive results from SEQUOIA-HCM, the pivotal Phase 3 clinical trial evaluating aficamten, a next-in-class cardiac myosin inhibitor, in obstructive hypertrophic cardiomyopathy (HCM), Cytokinetics submitted an NDA for aficamten to the U.S. Food & Drug Administration and is progressing regulatory submissions for aficamten for the treatment of obstructive HCM in Europe. Aficamten is also currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, CEDAR-HCM, a clinical trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM. Cytokinetics is also developing omecamtiv mecarbil, a cardiac muscle activator, in patients with heart failure with severely reduced ejection fraction (HFrEF), CK-586, a cardiac myosin inhibitor with a mechanism of action distinct from aficamten for the potential treatment of heart failure with preserved ejection fraction (HFpEF), and CK-089, a fast skeletal muscle troponin activator (FSTA) for the potential treatment of a specific type of muscular dystrophy.
For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on X, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act"). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act's Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, statements, express or implied, relating to the Company's development plans for omecamtiv mecarbil in the United States. Such statements are based on management's current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to, potential difficulties or delays in the development, testing, regulatory approvals for trial commencement, progression or product sale or manufacturing, or production of Cytokinetics' drug candidates that could slow or prevent clinical development or product approval; Cytokinetics' drug candidates may have adverse side effects or inadequate therapeutic efficacy; the FDA or foreign regulatory agencies may delay or limit Cytokinetics' ability to conduct clinical trials; Cytokinetics may be unable to obtain or maintain patent or trade secret protection for its intellectual property; standards of care may change, rendering Cytokinetics' drug candidates obsolete; and competitive products or alternative therapies may be developed by others for the treatment of indications Cytokinetics' drug candidates and potential drug candidates may target. For further information regarding these and other risks related to Cytokinetics' business, investors should consult Cytokinetics' filings with the Securities and Exchange Commission.
CYTOKINETICS® and the C-shaped logo are registered trademarks of Cytokinetics in the U.S. and certain other countries.
Contact:
Cytokinetics
Diane Weiser
Senior Vice President, Corporate Affairs
(415) 290-7757
References:
- Psotka MA, Gottlieb SS, Francis GS et al. Cardiac Calcitropes, Myotropes, and Mitotropes. JACC. 2019; 73:2345-53.
- Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J. et al. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat Commun. 2017;8:190.
- Shen YT, Malik FI, Zhao X, et al. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010; 3: 522-27.
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011 Mar 18;331(6023):1439-43.
- James et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Lancet 2018; 392: 1789–858.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200.
- Roger VL. Epidemiology of Heart Failure. Circulation Research. 2013;113:646-659, originally published August 29, 2013. doi: 10.1161/CIRCRESAHA.113.300268.
- Kilgore M, Patel HK, Kielhorn A, et al. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017; 10: 63-70.
- Taylor C J, Ordóñez-Mena J M, Roalfe A K, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ 2019; 364:l223 doi:10.1136/bmj.l223
- Greene SJ, Bauersachs J, Brugts JJ, et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. JACC. 2023 Jan 31;81(4):413-424. doi:10.1016/j.jacc.2022.11.023. PMID: 36697141.
- Extrapolated from Desai NR, Butler J, Binder G, et al. Prevalence and Excess Risk of Hospitalization in Heart Failure with Reduced Ejection Fraction. Poster presented at: Heart Failure Society of America (HFSA) Annual Scientific Meeting; 2022 Sep 30-Oct 3; Washington, DC.
- Carnicelli AP, Clare RM, Hofmann P, et al. Clinical trajectory of patients with a worsening heart failure event and reduced ventricular ejection fraction. Am Heart J. 2022 Mar; 245:110-116. doi: 10.1016/j.ahj.2021.12.003. Epub 2021 Dec 18. PMID: 34932997.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ee1006b4-fa47-4800-8929-5cdb837d546e


