Cytokinetics Presents Additional Data From SEQUOIA-HCM at the European Society of Cardiology Congress 2024
Additional Data from SEQUOIA-HCM Demonstrate Favorable Cardiac Remodeling
by Cardiac MRI, Improvements in Cardiac Structure and Function by Echocardiography,
Symptom Relief and Improvement in Biomarkers with Aficamten
Presentations Accompanied by Four Simultaneous Journal Publications
Company to Host Conference Call and Webcast
Tuesday, September 3rd at 8:00 AM Eastern Time
SOUTH SAN FRANCISCO, Calif., Sept. 01, 2024 (GLOBE NEWSWIRE) -- Cytokinetics, Incorporated (NASDAQ:CYTK) today announced that additional data from SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in HCM), the pivotal Phase 3 clinical trial of aficamten in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM), related to cardiac remodeling and improvements in patient symptoms, cardiac structure, function and biomarkers, were presented in three Late Breaking Clinical Trial presentations and one oral presentation at the European Society of Cardiology Congress 2024 in London, UK. These presentations were accompanied by simultaneous publications in leading cardiac journals, three in the Journal of the American College of Cardiology and one in the European Heart Journal.
"As we continue to dissect data from SEQUOIA-HCM, the expanding body of evidence reinforces the effects of aficamten on clinical outcomes, symptom burden, cardiac biomarkers and cardiac structure and function," said Stephen Heitner, M.D., Vice President, Head of Clinical Research. "Key data presented and published today show that treatment with aficamten appears to improve the architecture of the heart in patients with obstructive HCM, suggesting potential for disease modification. These data reinforce the primary analyses of SEQUOIA-HCM which are central to our rolling NDA submission for aficamten expected to be completed during this third quarter."
Data from SEQUOIA-HCM CMR Sub-Study Show Treatment with Aficamten is Associated with Favorable Cardiac Remodeling
Ahmad Masri, M.D., MS, Director of the Hypertrophic Cardiomyopathy Center at Oregon Health & Science University presented data from the cardiac magnetic resonance (CMR) sub-study in SEQUOIA-HCM. The data were simultaneously published in Journal of the American College of Cardiology.1 Of the 282 patients with obstructive HCM who participated in SEQUOIA-HCM, 57 patients participated in the CMR-sub-study and 50 patients completed the study, including 21 patients who received aficamten and 29 patients who received placebo. Baseline characteristics of patients enrolled in the CMR sub-study were comparable to the overall patient population in SEQUOIA-HCM. The primary endpoint in the CMR sub-study was the change from baseline to Week 24 in left ventricular mass index (LVMI) from baseline. Treatment with aficamten significantly improved LVMI (-15.4 g/m2, p=0.001) and resulted in favorable cardiac remodeling as demonstrated by reductions in left ventricular maximal wall thickness (p<0.001), left atrial volume index (LAVI) (p<0.001), and extracellular volume mass index (ECVi) (p=0.014), while replacement fibrosis (late gadolinium enhancement [LGE]) remained stable (Table 1). These observed structural changes are suggestive of ongoing favorable remodeling and occurred in conjunction with improvements in clinical endpoints including resting and Valsalva left ventricular outflow tract gradient (LVOT-G), NT-proBNP and NYHA Functional Class. A CMR sub-study is also being conducted in FOREST-HCM, the ongoing open-label extension clinical trial of aficamten, to evaluate cardiac remodeling associated with long-term treatment with aficamten, as well as in ACACIA-HCM, the pivotal Phase 3 clinical trial of aficamten in patients with non-obstructive HCM.
| Table 1: SEQUOIA-HCM CMR Sub-Study | ||||||
| Endpoint (SD) | Aficamten N=21 | Placebo N=29 | Treatment Effect (95% CI) | P-value | ||
| Baseline | Week 24 | Baseline | Week 24 | |||
| LV Mass Index (g/m2) | 113 ± 33 | 103 ± 28 | 110 ± 27 | 116 ± 33 | -15 (-25, -6) | <0.001 |
| LV Maximal Wall Thickness (mm) | 19.3 ± 4.3 | 17.8 ± 4.5 | 19.9 ± 4.4 | 20.4 ± 4.1 | -2.1 (-3.1, -1.1) | <0.001 |
| LA Volume Index (mL/m2) | 68 ± 19 | 52 ± 14 | 61 ± 16 | 60 ± 15 | -13 (-19, -7) | <0.001 |
| LV extracellular volume mass index (%/g/m2) | 31 ± 10 | 29 ± 12 | 30.7 ± 9.4 | 33 ± 11 | −3.9 (−7.0, −0.9) | 0.014 |
| LGE, (%) (IQR) | 2.0 (0.3, 3.8) | 1.4 (0.7, 2.2) | 2.5 (0.7, 7.4) | 1.7 (0.6, 3.8) | −0.4 (−2.1, 1.2) | 0.60 |
Aficamten Improves Echocardiographic Measures of Cardiac Structure and Function Without Negatively Impacting Systolic Function
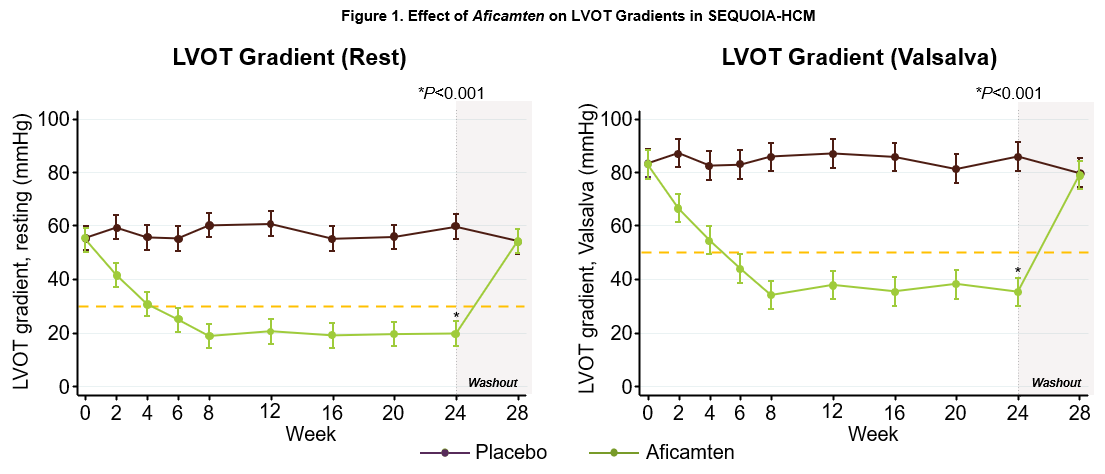
Sheila Hegde, M.D., M.P.H., Cardiovascular Medicine Specialist, Division of Cardiovascular Medicine, Brigham and Women's Hospital presented data from an analysis of the impact of treatment with aficamten on echocardiographic cardiac structure and function from SEQUOIA-HCM. The data were simultaneously published in Journal of the American College of Cardiology.2 As previously reported, treatment with aficamten significantly improved gradients by approximately 60%, improving placebo-corrected resting LVOT-G -40 mmHg (55 to 20 mmHg with aficamten) and Valsalva LVOT-G -50 mmHg (86 to 35 mmHg with aficamten) (Figure 1), with no significant adverse changes in left ventricular systolic function, as measured by left ventricular ejection fraction (LVEF) (-4.8%, 95% CI -6.4 to -3.3; p<0.001). Aficamten also improved measures of left ventricular structure and function including maximal wall thickness, septal wall thickness, inferolateral wall thickness, left ventricular mass index, and left ventricular end systolic volume index, with no change in left ventricular end diastolic volume index. Aficamten also improved LAVI as well as left ventricular relaxation and filling as indicated by an increase in lateral e' velocity and decrease in lateral E/e', findings consistent with an improvement in diastolic function (Table 2). The improvements in these echocardiographic measures were each associated with improvements in pVO2, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and NT-proBNP, demonstrating that the effects of aficamten on cardiac function and structure were associated with improvements in exercise capacity, symptoms and quality of life.
| Table 2. SEQUOIA-HCM: Echocardiographic Measures of Cardiac Structure and Function | ||
| Endpoint | Placebo-corrected treatment difference (95% CI) | P-value |
| Maximal Wall Thickness (mm) | -1.2 (-1.8, -0.6) | p<0.001 |
| Septal Wall Thickness (mm) | -1.0 (-1.6, -0.3) | p=0.003 |
| Inferolateral Wall Thickness (mm) | -0.8 (-1.3, -0.3) | p=0.003 |
| LV Mass Index (g/m2) | -12.2 (-18.0, -6.5) | p <0.001 |
| LV End Diastolic Volume Index (ml/m2) | -0.2 (-1.5 to 1.2) | p=0.8 |
| LV End Systolic Volume Index (ml/m2) | +1.7 (1.0, 2.4) | p<0.001 |
| LA Volume Index (ml/m2) | -3.8 (-5.5, -2.2) | p<0.001 |
| Lateral e' Velocity (cm/s) | +1.2 (0.7, 1.6) | p<0.001 |
| Lateral E/e' | -3.9 (-5.0, -2.0) | p<0.001 |
| Data are least square mean difference (95% confidence interval) | ||
Aficamten Improves Symptom Burden and Quality of Life in Patients with Obstructive HCM
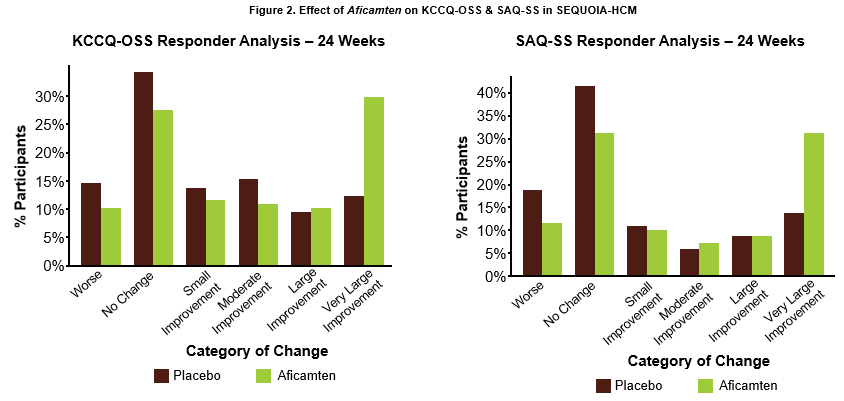
John A. Spertus, M.D., M.P.H., Professor, Daniel J. Lauer Missouri Endowed Chair in Metabolic and Vascular Disease Research, Clinical Director, University of Missouri Kansas City Healthcare Institute for Innovations in Quality and Saint Luke's Mid America Heart Institute presented results from an analysis of the effect of treatment with aficamten on patient symptom burden, including the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS) and the Seattle Angina Questionnaire Summary Score (SAQ-SS) from SEQUOIA-HCM. The data were simultaneously published in Journal of the American College of Cardiology.3 At 24 weeks, aficamten significantly improved KCCQ-OSS by 7.9 points (95% CI 4.8 to 11; p<0.001). Very large improvements in KCCQ-OSS (≥20 points) were achieved by 30% of patients treated with aficamten compared to 12% of patients treated with placebo (number needed to treat (NNT) = 5.8). Similarly, aficamten significantly improved SAQ-SS scores by 7.8 points (95% CI 4.7 to 11; p<0.001), with 31% of patients treated with aficamten experiencing a very large improvement (≥20 points) compared to 14% of patients on placebo (NNT = 5.8) (Figure 2). No significant heterogeneity was observed in improvements in KCCQ-OSS and SAQ-SS according to patient baseline characteristics, including the severity of disease or level of symptom burden. Even in patients with minimal angina at baseline, SAQ-SS scores improved after 24 weeks of treatment with aficamten. These results indicate that treatment with aficamten significantly improves patient health status including symptoms, function and quality of life.
| Table 2. SEQUOIA-HCM: Effect on Symptom Burden and Quality of Life | ||
| Endpoint | Mean Difference of Aficamten Compared to Placebo Change from Baseline to 24 Weeks | P-value |
| KCCQ-OSS | 7.9 (95% CI: 4.8, 11.0) | p<0.001 |
| SAQ-SS | 7.8 (95% CI: 4.7, 11.0) | p<0.001 |
| Data are least square mean difference (95% confidence interval) | ||
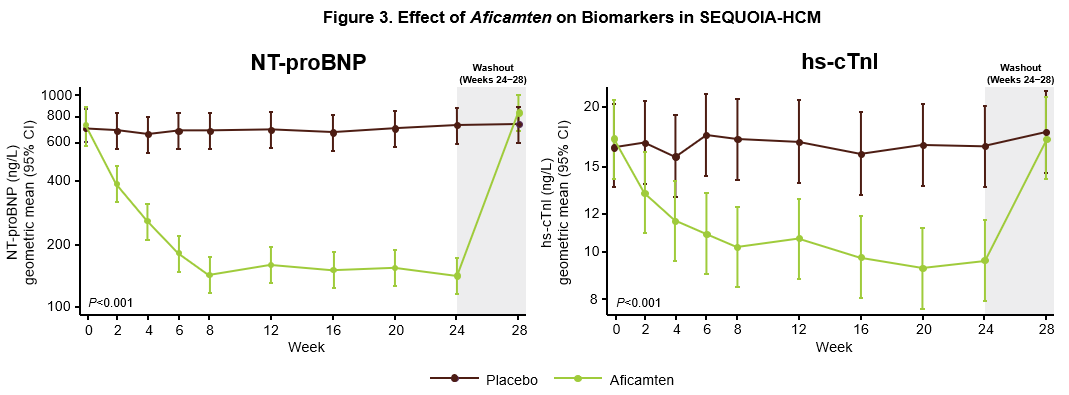
Aficamten Improves Cardiac Biomarkers Including NT-proBNP and hs-cTnI
Caroline Coats, M.D., Ph.D., Lead Clinician, West of Scotland Inherited Cardiac Conditions Service, Honorary Senior Lecturer, School of Cardiovascular and Metabolic Health, University of Glasgow presented results from a pre-specified secondary analysis from SEQUOIA-HCM related to NT-proBNP and high sensitivity cardiac troponin (hs-cTnI), cardiac biomarkers indicative of cardiac wall stress and myocardial injury. The results were simultaneously published in the European Heart Journal.4 Higher baseline concentrations of NT-proBNP and hs-cTnI were associated with worse baseline echocardiographic measures of disease severity including resting and Valsalva LVOT-G, LVEF and left ventricular wall thickness. Treatment with aficamten for 24 weeks resulted in an 80% reduction in NT-proBNP (p<0.001) and a 43% reduction (p<0.001) in hs-cTnI (Figure 3). Both measurements returned to baseline after washout. Baseline measurements of NT-proBNP and hs-cTnI were not correlated with change in pVO2 such that treatment with aficamten significantly improved pVO2 irrespective of baseline biomarkers. Baseline NT-proBNP and hs-cTnI also did not predict future instances of LVEF <50%. However, at 24 weeks, improvements in NT-proBNP and hs-cTnI were strongly associated with improvements in LVOT-G, pVO2 and KCCQ. Improvements in NT-proBNP were shown to closely mirror improvements in pVO2, which was not directly related to the reduction in LVOT-G, indicating that NT-proBNP may serve as an independent surrogate for change in pVO2. Additionally, changes in NT-proBNP as early as Week 2 were strongly associated with changes in Valsalva LVOT-G, KCCQ-CSS, LAVI and lateral E/e' at Week 24, indicating that early improvements in NT-proBNP may be a signal of future treatment effect. These results indicate that NT-proBNP may be a potentially useful tool to monitor functional and symptomatic improvements in response to treatment with aficamten.
Conference Call and Webcast
Cytokinetics will host a conference call on September 3, 2024 at 8:00 AM Eastern Time that will be simultaneously webcast and can be accessed from the Investors & Media section of Cytokinetics' website at www.cytokinetics.com. The live audio of the conference call can also be accessed by telephone by registering in advance at the following link: Cytokinetics ESC Investor Conference Call. Upon registration, participants will receive a dial-in number and a unique passcode to access the call. An archived replay of the webcast will be available via Cytokinetics' website for six months.
About Aficamten
Aficamten is an investigational selective, small molecule cardiac myosin inhibitor discovered following an extensive chemical optimization program that was conducted with careful attention to therapeutic index and pharmacokinetic properties and as may translate into next-in-class potential in clinical development. Aficamten was designed to reduce the number of active actin-myosin cross bridges during each cardiac cycle and consequently suppress the myocardial hypercontractility that is associated with hypertrophic cardiomyopathy (HCM). In preclinical models, aficamten reduced myocardial contractility by binding directly to cardiac myosin at a distinct and selective allosteric binding site, thereby preventing myosin from entering a force producing state.
The development program for aficamten is assessing its potential as a treatment that improves exercise capacity and relieves symptoms in patients with HCM as well as its potential long-term effects on cardiac structure and function. Aficamten was evaluated in SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in HCM), a positive pivotal Phase 3 clinical trial in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). Aficamten received Breakthrough Therapy Designation for the treatment of symptomatic obstructive HCM from the U.S. Food & Drug Administration (FDA) as well as the National Medical Products Administration (NMPA) in China. Cytokinetics expects to submit a New Drug Application (NDA) to the FDA in Q3 2024 and a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in Q4 2024.
Aficamten is also currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, and CEDAR-HCM, a clinical trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM.
About Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a disease in which the heart muscle (myocardium) becomes abnormally thick (hypertrophied). The thickening of cardiac muscle leads to the inside of the left ventricle becoming smaller and stiffer, and thus the ventricle becomes less able to relax and fill with blood. This ultimately limits the heart's pumping function, resulting in reduced exercise capacity and symptoms including chest pain, dizziness, shortness of breath, or fainting during physical activity. HCM is the most common monogenic inherited cardiovascular disorder, with approximately 280,000 patients diagnosed, however, there are an estimated 400,000-800,000 additional patients who remain undiagnosed in the U.S.5,6,7 Two-thirds of patients with HCM have obstructive HCM (oHCM), where the thickening of the cardiac muscle leads to left ventricular outflow tract (LVOT) obstruction, while one-third have non-obstructive HCM (nHCM), where blood flow isn't impacted, but the heart muscle is still thickened. People with HCM are at high risk of also developing cardiovascular complications including atrial fibrillation, stroke and mitral valve disease.8 People with HCM are at risk for potentially fatal ventricular arrhythmias and it is one of the leading causes of sudden cardiac death in younger people or athletes.9 A subset of patients with HCM are at high risk of progressive disease leading to dilated cardiomyopathy and heart failure necessitating cardiac transplantation.
About Cytokinetics
Cytokinetics is a late-stage, specialty cardiovascular biopharmaceutical company focused on discovering, developing and commercializing muscle biology-directed drug candidates as potential treatments for debilitating diseases in which cardiac muscle performance is compromised. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact myocardial muscle function and contractility. Cytokinetics is preparing for regulatory submissions for aficamten, its next-in-class cardiac myosin inhibitor, following positive results from SEQUOIA-HCM, the pivotal Phase 3 clinical trial in obstructive hypertrophic cardiomyopathy which were published in the New England Journal of Medicine. Aficamten is also currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, CEDAR-HCM, a clinical trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM. Cytokinetics is also developing omecamtiv mecarbil, a cardiac muscle activator, in patients with heart failure. Additionally, Cytokinetics is developing CK-586, a cardiac myosin inhibitor with a mechanism of action distinct from aficamten for the potential treatment of HFpEF.
For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on X, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act"). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act's Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, statements express or implied relating to the properties or potential benefits of aficamten or any of our other drug candidates, our ability to obtain regulatory approval for aficamten for the treatment of obstructive hypertrophic cardiomyopathy or any other indication from FDA or any other regulatory body in the United States or abroad, and the labeling or post-marketing conditions that FDA or another regulatory body may require in connection with the approval of aficamten. Such statements are based on management's current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to the risks related to Cytokinetics' business outlines in Cytokinetics' filings with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and Cytokinetics' actual results of operations, financial condition and liquidity, and the development of the industry in which it operates, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that Cytokinetics makes in this press release speak only as of the date of this press release. Cytokinetics assumes no obligation to update its forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
CYTOKINETICS® and the C-shaped logo are registered trademarks of Cytokinetics in the U.S. and certain other countries.
Contact:
Cytokinetics
Diane Weiser
Senior Vice President, Corporate Affairs
(415) 290-7757
References:
- Masri A, et al. Effect of Aficamten on Cardiac Structure and Function in Obstructive Hypertrophic Cardiomyopathy: SEQUOIA-HCM CMR Substudy. JACC. 2024.
- Hegde S, et al. Impact of Aficamten on Echocardiographic Cardiac Structure and Function in Symptomatic Obstructive Hypertrophic Cardiomyopathy. JACC. 2024.
- Sherrod C, et al. Effect of Aficamten on Health Status Outcomes in Obstructive Hypertrophic Cardiomyopathy: Results from SEQUOIA-HCM. JACC. 2024.
- Coats CJ, et al. Cardiac Biomarkers and Effects of Aficamten in Obstructive Hypertrophic Cardiomyopathy: The SEQUOIA-HCM Trial. Eur Heart J. 2024
- CVrg: Heart Failure 2020-2029, p 44; Maron et al. 2013 DOI: 10.1016/S0140-6736(12)60397-3; Maron et al 2018 10.1056/NEJMra1710575
- Symphony Health 2016-2021 Patient Claims Data DoF;
- Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R, Olivotto I. Occurrence of Clinically Diagnosed Hypertrophic Cardiomyopathy in the United States. Am J Cardiol. 2016; 15;117(10):1651-1654.
- Gersh, B.J., Maron, B.J., Bonow, R.O., Dearani, J.A., Fifer, M.A., Link, M.S., et al. 2011 ACCF/AHA guidelines for the diagnosis and treatment of hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Journal of the American College of Cardiology and Circulation, 58, e212-260.
- Hong Y, Su WW, Li X. Risk factors of sudden cardiac death in hypertrophic cardiomyopathy. Current Opinion in Cardiology. 2022 Jan 1;37(1):15-21
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/a4d56c0a-6c2f-41a3-b475-8292c7cf0b2c
https://www.globenewswire.com/NewsRoom/AttachmentNg/91ea85c4-94d8-42f9-a233-28c283093cd0
https://www.globenewswire.com/NewsRoom/AttachmentNg/cbb0ded2-902e-4365-8d5b-14efaf733407


