Dexcom Unveils New Report on Type 2 Diabetes Management in the U.S. at ADA 2025
- New State of Type 2 Report in the United States reports that a majority of healthcare professionals (HCPs) surveyed believe continuous glucose monitoring will have the potential to be more impactful on the future of Type 2 diabetes care than advancements in diabetes medications.1
- Dexcom Warrior Lance Bass will share first-hand experience saying "Bye" to fingersticks* during panel at ADA.
DexCom, Inc., the global leader in glucose biosensing, today released its "Dexcom State of Type 2 Report: Access and Attitudes Across the United States" ahead of the 85th Scientific Sessions of the American Diabetes Association (ADA) in Chicago. The findings provide valuable insights into the perceptions around diabetes technology from more than 400 healthcare professionals and people with Type 2 diabetes across the United States.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250618841089/en/
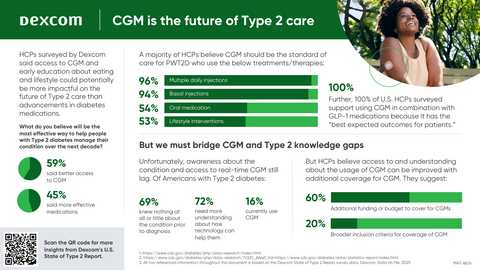
New State of Type 2 Report in the United States reports that a majority of HCPs surveyed believe continuous glucose monitoring will have the potential to be more impactful on the future of Type 2 diabetes care than advancements in diabetes medications.
During the conference, Dexcom will present extensive clinical data that shows the benefits of CGM for those living with Type 2 diabetes as well as new outcomes from early Stelo users.
Dexcom releases new State of Type 2 Report: Access and Attitudes Across the United States
Earlier this year, Dexcom announced the release of its first multi-region report, detailing access and attitudes of individuals diagnosed with Type 2 diabetes and healthcare professionals across Europe and the Middle East (EMEA). The latest State of Type 2 Report now builds on those initial findings with data collected in the U.S. Topline takeaways include:
- Tech is believed to be an effective way to manage Type 2 diabetes: More than half (59%) of U.S. HCPs believe that better access to CGM will be the most effective way to help people with Type 2 diabetes manage their condition over the next decade.1
- U.S. HCPs support CGM as "standard of care": 96% of providers surveyed in the U.S. agree that CGM should be the standard of care for individuals using multiple daily insulin injections, and 94% agree that CGM should be the standard of care for those on basal insulin.1
- U.S. HCPs unanimously support use of CGM in combination with GLP-1 medications: 100% of U.S. HCPs surveyed recommend CGM in combination with GLP-1 or SGLT2 medication because it has the best expected outcomes for patients, and 79% of providers in EMEA agreed.1
- Better coverage, more education and training can improve understanding of CGM: HCPs believe access and understanding about the usage of CGM can be improved with additional coverage and more educational support for Americans with Type 2 diabetes, as well as more training for healthcare providers. Specifically, 60% of U.S. HCPs said additional funding or budget to cover CGM could increase usage among people with Type 2 diabetes.1
"The findings of our State of Type 2 Report in the U.S. reaffirm what we've always believed to be true: CGM is central to the future of Type 2 diabetes care," said Jake Leach, president and chief operating officer of Dexcom. "To help drive the greatest impact for patients, a continued focus on diabetes education and access to CGMs is needed – and Dexcom is committed to advancing these around the world."
Read the full Dexcom State of Type 2 Report here: https://provider.dexcom.com/future-type-2-diabetes-care
Dexcom presents robust clinical data and hosts compelling presentations
Conference attendees will have the opportunity to hear firsthand from Grammy-nominated singer, actor, producer and Dexcom Warrior Lance Bass about how Dexcom CGM has helped him live his best life during Dexcom's product theater on Saturday, June 21.
After being diagnosed with Latent Autoimmune Diabetes in Adults (LADA), also known as Type 1.5 diabetes, Lance has been an advocate for the diabetes community, saying ‘Bye' to fingersticks*, guesswork and uncertainty with the help of Dexcom G7 technology. Lance will be joined by experts who will also speak about how early adoption of CGM can improve diabetes management through personalized care in addition to innovations in Dexcom technology, including new features and in-app reports.
Additionally, Dexcom will present clinical data at the conference that continues to support the benefits of its technology for people with diabetes of all ages and stages. Most notably, several studies looking at CGM use for those with Type 2 diabetes across various insulin therapies show numerous benefits – adding to an already extensive body of evidence – from reduced mortality risk among insulin-using people2 to reduced diabetes-related distress and improved self-management3 among non-insulin using people.
In a real-world observational study of individuals with Type 2 diabetes not using insulin, Dexcom G7 use was associated with significantly reduced diabetes-related distress and increased adherence to healthy eating plans and exercise routines, supporting CGM as a powerful tool for behavior modification.3
For a detailed overview of all Dexcom presentations at ADA this year, visit: https://professional.diabetes.org/scientific-sessions.
About DexCom, Inc.
Dexcom empowers people to take control of health through innovative biosensing technology. Founded in 1999, Dexcom has pioneered and set the standard in glucose biosensing for more than 25 years. Its technology has transformed how people manage diabetes and track their glucose, helping them feel more in control and live more confidently.
Dexcom. Discover what you're made of. For more information, visit www.dexcom.com.
Category: IR
____________________ |
*Fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
1 Dexcom State of Type 2 Report (US), Dexcom data on file, 2025. 2 Blake CL, et al. "Reduced Mortality Risk Associated with Dexcom rtCGM Compared to SMBG Among People with T2D on Any Insulin Therapy." Presented at ADA 2025. 3 Crawford, MA, et al. "Real-World Dexcom CGM Use in T2D NIT: Reduced Diabetes Distress and Improved Self-Care Behaviors." Presented at ADA 2025.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250618841089/en/
Media Relations Contact
Nadia Conard
[email protected]
Investor Relations Contact
Sean Christensen
[email protected]
