FDA Approves Expanded Indications for GE HealthCare's Vizamyl PET Imaging Agent for Beta Amyloid Detection, Enabling More Precise Care for Alzheimer's Patients
- The FDA has approved an updated label for Vizamyl (flutemetamol F 18 injection), including quantification which enables a more continuous and objective measure of amyloid in the brain
- This approval also enables clinicians to use Vizamyl to monitor patient response to anti-amyloid therapies
- Other updates include now enabling the use of Vizamyl to predict development of dementia or other cognitive decline due to Alzheimer's disease, selecting patients who are indicated for anti-amyloid therapies, and establishing a diagnosis of Alzheimer's disease.
- The updated label enables enhanced decision making, and more confident diagnosis and monitoring of Alzheimer's disease, helping patients and their families access timely, precision care.
GE HealthCare (NASDAQ:GEHC) today announced that the U.S. Food and Drug Administration (FDA) has approved an updated label for its positron emission tomography (PET) imaging agent VizamylTM (flutemetamol F 18 injection) for beta-amyloid detection. The revised label, effective immediately, expands the indications for use, enables quantitative analysis of Vizamyl scans, and removes significant previous limitations such as monitoring patient response to anti-amyloid therapy.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250624101667/en/
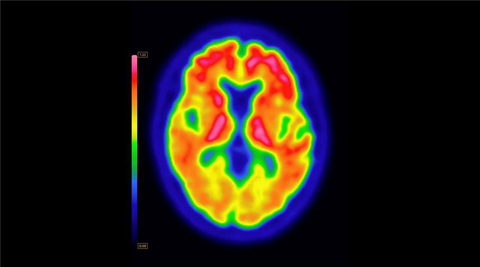
PET brain scan using Vizamyl (flutemetamol F18) with quantification software
Up to now, amyloid diagnostics such as Vizamyl have been used to provide a visual assessment of amyloid plaque accumulation in the brain. With quantification now added to the label, clinicians can reach a more objective assessment, using software that enables a calculation of amyloid load, with published research demonstrating that quantification improves diagnostic confidence and consistency among readers1,2,3. In addition, with the removal of a limitation of use for monitoring therapy effectiveness, Vizamyl can also now be used to assess whether the level of amyloid plaques has been reduced sufficiently for the therapy to potentially be stopped.
"The inclusion of quantification and removal of the therapy monitoring limitation from the Vizamyl label is good news for healthcare providers and their patients, further enabling timely and appropriate care decisions," said Jit Saini, MD, Chief Medical Officer of the Pharmaceutical Diagnostics (PDx) division of GE HealthCare. "These changes pave the way for clinicians to expand their usage of Vizamyl, with meaningful implications for patients and their families— helping provide clearer answers, earlier diagnoses, and enabling more personalized treatment strategies."
"The use of quantification in amyloid PET imaging has steadily moved from research to clinical practice, where it can aid in more confident and accurate diagnosis," said Phillip Kuo, MD, PhD, FACR, Professor of Radiology, Section Chief of Nuclear Medicine and Director Theranostics at City of Hope National Medical Center. "Now quantification can also play a critical role in initiating and monitoring amyloid-targeted therapy for Alzheimer's disease and determining when it can be discontinued."
The label update also adds an explicit indication for selection of patients eligible for therapy and removes several previous limitations of use, including for the diagnosis of Alzheimer's disease, based on revised criteria from the Alzheimer's Association, indicating that an abnormal amyloid PET scan is sufficient to establish a diagnosis. Furthermore, the label now removes a previous limitation on predicting the cognitive decline or progression to dementia, based on evidence linking amyloid-positive scans to a higher risk of progression from the early mild cognitive impairment phase of Alzheimer's dementia4.
Vizamyl was first approved in 2013 to estimate beta amyloid neuritic plaque density in adult patients with cognitive impairment. GE HealthCare offers solutions for quantitative analysis of amyloid PET scans including through its MIM Neuro Software platform which has been recently FDA cleared for centiloid scaling.
GE HealthCare's Pharmaceutical Diagnostics division is a global leader in imaging agents used to support around 130 million procedures per year globally, equivalent to four patient procedures every second. Its Molecular Imaging portfolio combines established proprietary products across cardiology, neurology and oncology, with an innovative pipeline, all aimed at enabling better informed diagnosis and monitoring for improved therapy decision making and clinical outcomes.
Learn more about Vizamyl at GE HealthCare's PDx booth 1029 at the SNMMI 2025 Annual Meeting June 21 – 24 in New Orleans, LA or online here: https://www.gehealthcare.com/products/molecular-imaging-agents/vizamyl.
About GE HealthCare Technologies Inc.
GE HealthCare is a trusted partner and leading global healthcare solutions provider, innovating medical technology, pharmaceutical diagnostics, and integrated, cloud-first AI-enabled solutions, services and data analytics. We aim to make hospitals and health systems more efficient, clinicians more effective, therapies more precise, and patients healthier and happier. Serving patients and providers for more than 125 years, GE HealthCare is advancing personalized, connected and compassionate care, while simplifying the patient's journey across care pathways. Together, our Imaging, Advanced Visualization Solutions, Patient Care Solutions and Pharmaceutical Diagnostics businesses help improve patient care from screening and diagnosis to therapy and monitoring. We are a $19.7 billion business with approximately 53,000 colleagues working to create a world where healthcare has no limits.
GE HealthCare is proud to be among 2025 Fortune World's Most Admired Companies™.
Follow us on LinkedIn, X, Facebook, Instagram, and Insights for the latest news, or visit our website https://www.gehealthcare.com for more information.
The Alzheimer's Association makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Important Safety Information for VizamylTM (flutemetamol F18) injection
PRODUCT INDICATIONS AND USE
VIZAMYL™ (flutemetamol F 18 injection) is indicated for positron-emission tomography (PET) of the brain to estimate amyloid beta neuritic plaque density in adults with cognitive impairment for:
- Evaluation of Alzheimer's disease (AD) and other causes of cognitive decline
- Selection of patients who are indicated for amyloid beta-directed therapy as described in the prescribing of information of the therapeutic products.
CONTRAINDICATIONS
VIZAMYL is contraindicated in patients with a history of hypersensitivity reaction to VIZAMYL or polysorbate 80.
WARNINGS AND PRECAUTIONS
Anaphylaxis and Other Serious Hypersensitivity Reactions: Serious hypersensitivity reactions including anaphylaxis, presenting with flushing, dyspnea, and hypotension have been observed within minutes following administration and may occur in patients with no history of exposure to VIZAMYL. Obtain a history of allergy or hypersensitivity reactions. Always have resuscitation equipment and trained personnel immediately available at the time of VIZAMYL administration. If a hypersensitivity reaction is suspected, immediately discontinue the injection and initiate appropriate therapy. VIZAMYL is contraindicated in patients with a history of hypersensitivity to VIZAMYL or polysorbate 80.
Risk of Image Misinterpretation and Other Errors: Errors may occur in the estimation of amyloid beta neuritic plaque density during VIZAMYL image interpretation. The use of clinical information in the interpretation of VIZAMYL images has not been evaluation and may lead to an inaccurate assessment. Extensive brain atrophy and motion artifacts that distort the image may limit the ability to distinguish gray and white matter on a VIZAMYL scan. Perform image interpretation independently of the patient's clinical information. For cases where there is uncertainty as to the location of cortical signal, use co-registered anatomical imaging to improve localization of signal or examine the striatum for VIZAMYL signal as it is less affected by atrophy.
Radiation Risk: VIZAMYL contributes to a patient's long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe drug handling to protect patients and health care providers from unintentional radiation exposure. Advise patients to hydrate before and after administration and to void frequently after administration.
ADVERSE REACTIONS
The most commonly reported adverse reactions in clinical trials were flushing (2 %), increased blood pressure (2 %), headache (1 %), nausea and dizziness (1 %). Postmarketing experience included anaphylactic reactions. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
USE IN SPECIFIC POPULATIONS
Pregnancy: There are no available data on VIZAMYL in pregnant woman to evaluate drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. All radiopharmaceuticals, including VIZAMYL, have the potential to cause fetal harm depending on the stage of fetal development and the magnitude of the radiation dose. If considering VIZAMYL administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from the drug and the gestational timing of exposure.
Lactation: There are no data on the presence of flutemetamol F 18 or metabolites in human milk or its effects on the breastfed infant or milk production. Exposure of VIZAMYL to a breastfed infant can be minimized by temporary discontinuation of breastfeeding. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VIZAMYL and any potential adverse effects on the breastfed child from VIZAMYL or from the underlying maternal condition.
Pediatric Use: The safety and effectiveness of VIZAMYL have not been established pediatric patients.
Geriatric Use: No overall differences in safety or effectiveness were observed between subjects 65 years of age and older and younger adult subjects.
OVERDOSAGE
The major risks of overdosage relate predominantly to increased radiation exposure, with long-term risk for neoplasia. In the event of administration of a radiation overdose with VIZAMYL, hydration and frequent urination should be encouraged to minimize radiation exposure to the subject. It is unknown whether or not the flutemetamol is dialyzable.
Prior to VIZAMYL administration, please read the full Prescribing Information for additional Important Safety Information.
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 800 654 0118 (option 2 then option 1) or by email at [email protected] or FDA at 800 FDA 1088 or www.fda.gov/medwatch
1 Collij LE, Bischof GN, Altomare D et al. Quantification Supports Amyloid PET Visual Assessment of Challenging Cases: Results from the AMYPAD Diagnostic and Patient Management Study. J Nucl Med. 2025 Jan 3;66(1):110-116.
2 Collij LE, Salvadó G, Shekari M et al. Visual assessment of [(18)F]flutemetamol PET images can detect early amyloid pathology and grade its extent. Eur J Nucl Med Mol Imaging. 2021 Jul;48(7):2169-2182.
3 Bucci M, Savitcheva I, Farrar G et al, A multisite analysis of the concordance between visual image interpretation and quantitative analysis of [(18)F]flutemetamol amyloid PET images. Eur J Nucl Med Mol Imaging. 2021 Jul;48(7):2183-2199.
4 https://www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/mild-cognitive-impairment, accessed on June 19, 2025
View source version on businesswire.com: https://www.businesswire.com/news/home/20250624101667/en/
GE HealthCare Media Contact:
Emmy Elguizaoui
US Communications Director, PDx
GE HealthCare
+1 978 243 7503
[email protected]
