Fractyl Health Announces Groundbreaking Data from REMAIN-1 Midpoint Cohort Showing Revita® Maintained Weight Loss After GLP-1 Discontinuation
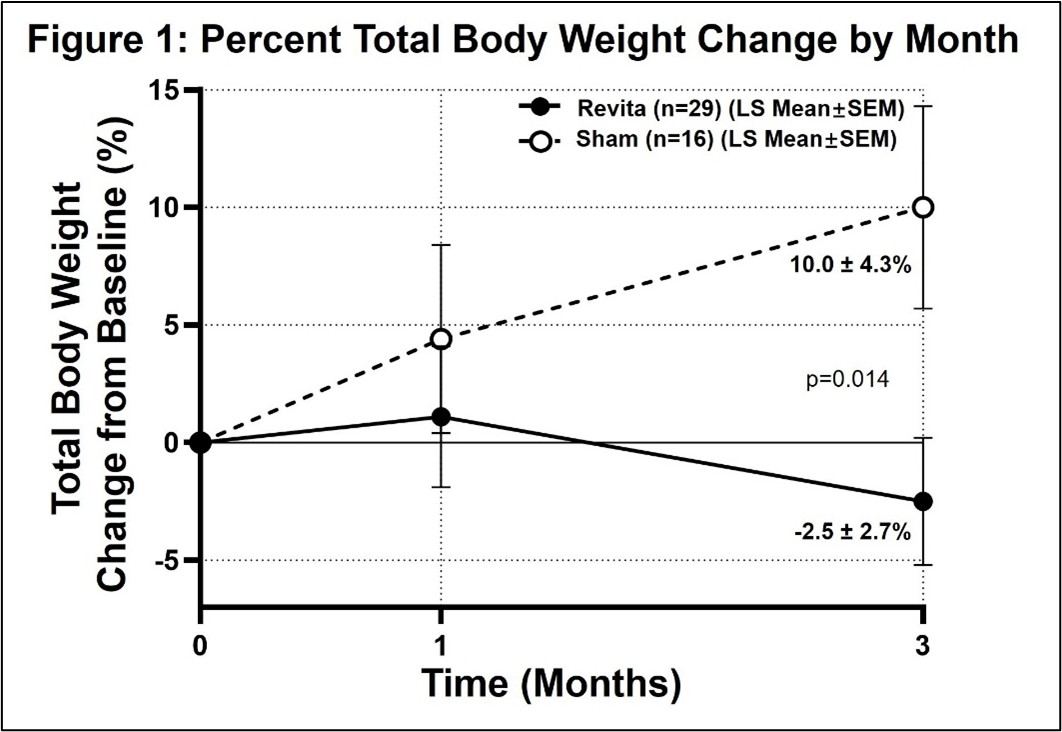
Pilot study met key 3-month efficacy endpoint with strong statistical significance (p=0.014); Revita-treated patients lost an additional 2.5% total body weight after stopping GLP-1 drugs vs. 10% regain in sham-treated patients
Revita procedure demonstrated excellent safety and tolerability to date, consistent with prior clinical studies
Results validate pivotal study design and reinforce Revita's potential to be the first therapy for post-GLP-1 weight maintenance
Company to host investor call and webcast today at 8:00 a.m. ET
BURLINGTON, Mass., Sept. 26, 2025 (GLOBE NEWSWIRE) -- Fractyl Health, Inc. (NASDAQ: GUTS) (the Company or Fractyl), a metabolic therapeutics company focused on pattern breaking approaches that treat root causes of obesity and type 2 diabetes (T2D), today announced groundbreaking results from the REMAIN-1 Midpoint Cohort, supporting the potential for Revita to be the first therapy to preserve weight loss after GLP-1 drug discontinuation. At 3 months, Revita-treated patients lost an additional 2.5% total body weight after stopping tirzepatide, while sham patients regained 10% (p=0.014). These results are clinically and statistically significant and provide randomized, blinded evidence that drug-free, durable weight maintenance is possible. They further support Revita as a potential first-in class treatment in a new therapeutic category in obesity care: post-GLP-1 weight maintenance.
"One of the biggest challenges in obesity medicine today is what happens when patients stop GLP-1 therapy, whether because of cost, insurance coverage, or side effects. We know weight regain almost always follows," said Shelby Sullivan, M.D., Professor of Medicine at the Geisel School of Medicine at Dartmouth University. "The REMAIN-1 3-month results are the first randomized, controlled evidence suggesting that a one-time endoscopic procedure may help maintain drug-free weight loss after GLP-1 discontinuation. The treatment difference we saw in this study, with Revita patients continuing to lose weight while sham patients rapidly regained weight, is striking. If these findings continue to hold with more data, duodenal mucosal resurfacing could represent a novel and much-needed solution to the largest gap in obesity care: post-GLP-1 weight maintenance."
REMAIN-1 Midpoint Cohort Study Design
The REMAIN-1 Midpoint Cohort (N=45) is a randomized, double-blind, sham-controlled study designed to evaluate Revita in adults with obesity who achieved at least 15% total body weight loss with tirzepatide. After discontinuing the drug, participants were randomized 2:1 to Revita or a sham endoscopic procedure. The key efficacy endpoint was total body weight change in Revita versus sham at 3 months.
The Midpoint Cohort is designed to be identical to the ongoing Pivotal Cohort, serving as an early readout to reinforce confidence in the pivotal program and provide initial validation of the study design and endpoints.
Key Findings
Data shared are for the treatment period of up to 3 months. The Midpoint Cohort is ongoing, with 6-month randomized data expected in Q1 2026.
- Clear evidence of Revita activity: The study met its 3-month efficacy endpoint with strong statistical significance (p=0.014), delivering 2.5% further weight loss with Revita (n=29) even after stopping tirzepatide, versus 10% weight regain in sham-treated patients (n=16).
- Excellent safety and tolerability through 3 months: No Revita-related SAEs or Grade II+ AEs were observed. Side effects were infrequent, mild, and transient, consistent with prior Revita clinical study experience.
- Positive readthrough to pivotal study: These data strengthen confidence in the ongoing Pivotal Cohort study, which is on track to complete randomization in early 2026, with 6-month topline primary endpoint data and potential PMA filing expected in H2 2026.
3-Month Post-Procedure Total Body Weight Change from Baseline (%)
"These results are a defining milestone for Fractyl and the obesity community. For the first time, randomized, blinded data show that Revita may dramatically prevent weight regain after GLP-1 discontinuation at 3 months. This is a compelling demonstration that targeting the gut may provide a remarkable and clinically significant improvement in obesity," said Harith Rajagopalan, M.D., Ph.D., Co-Founder and Chief Executive Officer of Fractyl Health. "The implications are profound. Patients want durable weight loss without the need for chronic medical therapy. The Revita clinical profile in this study suggests we have the potential to create a new standard of care in obesity with a disease-modifying intervention that allows us to progress from chasing weight loss to sustaining health with a durable metabolic reset."
Upcoming Milestones
The REMAIN-1 Midpoint Cohort is ongoing, with 6-month data expected in Q1 2026. The REMAIN-1 Pivotal Cohort has completed enrollment, and the Company remains on track to randomize patients in early 2026 and deliver 6-month topline primary endpoint data and potentially file a PMA in H2 2026. These milestones are designed to evaluate Revita as the first potential therapy for post-GLP-1 weight maintenance and to open a new therapeutic category in obesity care.
Webcast Information
Fractyl will host a live webcast today, Friday, September 26, 2025, at 8:00 a.m. ET, to discuss the REMAIN-1 Midpoint Cohort data. The live event can be accessed in the "Events" section of Fractyl's website at ir.fractyl.com. The webcast will be archived and available for replay for at least 30 days after the event.
About Fractyl Health
Fractyl Health is a metabolic therapeutics company focused on pioneering new approaches to the treatment of metabolic diseases, including obesity and T2D. Despite advances in treatment over the last 50 years, obesity and T2D continue to be rapidly growing drivers of morbidity and mortality in the 21st century. Fractyl's goal is to transform metabolic disease treatment from chronic symptomatic management to durable disease-modifying therapies that target the organ-level root causes of disease. The Company has a robust and growing IP portfolio, with 33 granted U.S. patents and approximately 40 pending U.S. applications, along with numerous foreign issued patents and pending applications. Fractyl is based in Burlington, MA. For more information, visit www.fractyl.com.
About Revita®
Fractyl Health's lead product candidate, Revita, is based on the company's insights surrounding the potential role of the gut in obesity. Revita is designed to remodel the duodenal lining via hydrothermal ablation (i.e. duodenal mucosal resurfacing) to reverse damage to intestinal nutrient sensing and signaling mechanisms caused by chronic high-fat and high-sugar diets that are a root cause of metabolic disease. In the U.S., Revita is for investigational use only under U.S. law. Revita has U.S. FDA Breakthrough Device designation in weight maintenance for people with obesity who discontinue GLP-1 drugs. A pivotal study of Revita in patients with obesity after discontinuation of GLP-1 drugs, called REMAIN-1, was initiated in the third quarter of 2024 and has completed enrollment.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact are forward-looking statements. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will" and variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements in this press release include, without limitation, statements regarding the promise and potential impact of our product candidates, including Revita's potential for preserving weight loss after GLP-1 drug discontinuation; the design, initiation, timing and results of clinical enrollment and any clinical studies or readouts, including readouts from the REMAIN-1 Midpoint Cohort; the potential treatment population or benefits for any of our product candidates or products; our strategic and product development objectives and goals, including with respect to enabling long-term control over obesity and type 2 diabetes without the burden of chronic therapies, redefining the future of metabolic disease treatment, and positioning our Company at the forefront of the global opportunity for metabolic care or a late-stage, pre-commercial company poised to redefine metabolic care; and the timing of any of the foregoing. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the Company's limited operating history; the incurrence of significant net losses and the fact that the Company expects to continue to incur significant net losses for the foreseeable future; the Company's need for substantial additional financing; the Company's ability to continue as a going concern; the restrictive and financial covenants in the Company's credit agreement; the lengthy and unpredictable regulatory approval process for the Company's product candidates; uncertainty regarding its clinical studies; the fact that the Company's product candidates may cause serious adverse events or undesirable side effects or have other properties that may cause it to suspend or discontinue clinical studies, delay or prevent regulatory development, prevent their regulatory approval, limit the commercial profile, or result in significant negative consequences; the Company's reliance on third parties to conduct certain aspects of the Company's preclinical studies and clinical studies; the Company's reliance on third parties for the manufacture of sub-assembly components for Revita; the regulatory approval process of the FDA and comparable foreign regulatory authorities is lengthy, time-consuming and inherently unpredictable, and even if we complete the necessary clinical studies, we cannot predict when, or if, we will obtain regulatory approval or certification for any of our product candidates, and any such regulatory approval or certification may be for a more narrow indication than we seek; and the potential launch or commercialization of any of Company's product candidates or products and our strategic and product development objectives and goals, and the other factors discussed under the caption "Risk Factors" in our Annual Report on Form 10-K for the year ended December 31, 2024 and Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, filed with the Securities and Exchange Commission on August 12, 2025 and in our other filings with the SEC. These forward-looking statements are based on management's current estimates and expectations. While the Company may elect to update such forward-looking statements at some point in the future, the Company disclaims any obligation to do so, even if subsequent events cause its views to change.
Contacts
Media Contact
Jessica Cotrone, Head of Corporate Communications
[email protected], 978.760.5622
Investor Contact
Brian Luque, Head of Investor Relations and Corporate Development
[email protected], 951.206.1200
An infographic accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d3719876-ad7c-482c-b847-ac1a6091839f


