IceCure's Cryoablation System Combined with Radiation Therapy Successfully Treats Non-Small Cell Lung Cancer (NSCLC) with 92% Disease-Specific 5-year Survival
5-year overall survival (OS) rate for stereotactic body radiation therapy (SBRT) followed by cryoablation was 74%, compared to published studies reporting 5-year OS of 41% - 52% with SBRT alone
SBRT followed by cryoablation produced 5-year OS outcomes similar to surgery, which has a 5-year OS of 67% - 82% according to published studies
Lung cancer is the most frequently diagnosed cancer worldwide with an incidence of 2.5 million cases and remains the leading cause of cancer-related death
CAESAREA, Israel, Nov. 3, 2025 /PRNewswire/ -- IceCure Medical Ltd. (NASDAQ:ICCM) ("IceCure", "IceCure Medical" or the "Company"), developer of minimally-invasive cryoablation technology that destroys tumors by freezing as an option to surgical tumor removal, today announced the publication of an independent study using IceCure's Cryoablation System titled "Long-term outcomes of combination therapy with stereotactic body radiation therapy plus cryoablation using liquid nitrogen for stage I non-small cell lung cancer with tumors ≥2 cm" in the peer-reviewed journal PLOS One. The study was led by Dr. Hiroaki Nomori of the Department of Thoracic Surgery, Kashiwa Kousei General Hospital, Japan, along with researchers from Tokyo University Hospital, Kashiwa Kousei General Hospital, and Sonodakai Radiation Clinic, Tokyo.
"While radiation therapy is the standard of care for inoperable stage I NSCLC patients, using SBRT alone unfortunately results in far lower overall survival and lower local control than surgery in certain patients. This study, which focused on people with relatively larger tumors, indicative of later stage disease, provides very encouraging results confirming that combining SBRT with our cryoablation system offers inoperable patients longer life expectancy and may also provide a minimally invasive option to surgery for the broader population of stage I NSCLC patients," stated IceCure's Chief Executive Officer, Eyal Shamir. "The results of this study may be highly impactful in our major markets including the U.S. and Europe."
The objective of the independent retrospective observational study was to evaluate the effectiveness of combining SBRT with cryoablation for treating stage I NSCLC tumors ≥2 cm, given the limitations of local control and survival rates with SBRT monotherapy. 64 patients with tumors of mean diameter of 2.7 ± 0.5 cm and a range of 2.0–4.0 cm were treated with SBRT, followed by cryoablation. The median follow-up duration was 74 months, with a range of 3-111 months.
Results include the following:
- 5-Year Local Control Rate: 93%
- 5-Year OS Rate: 74% compared to published studies which reported 5-year OS rates of 41% - 52% after SBRT alone for stage I NSCLC, including tumors <2 cm; while surgery, the standard treatment for stage I (IA and IB) NSCLC, has a 5-year OS of 67% - 82% according to published studies
- 3-Year Disease-specific survival: 96%
- 5-Year Disease-specific survival: 92%
- Treatment-Related Mortality: None
- Most frequent complications post-cryoablation: pneumothorax, CTCAE grade 2, 40%
The results of this study align with prior findings from independent studies, including a prior study by Nomuri et al. which reported a recurrence-free rate of 67% - 100% in lung cancer patients treated with IceCure's cryoablation system.
According to a study published in the CA: A Cancer Journal for Clinicians, the flagship journal of the American Cancer Society, lung cancer was the most frequently diagnosed cancer in 2022, responsible for almost 2.5 million new cases, or one in eight cancers worldwide (12.4% of all cancers) followed by breast cancer (11.6% of all cancers globally).
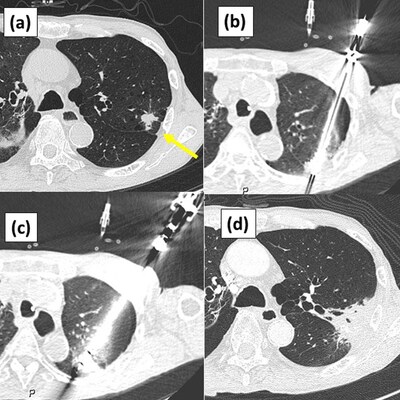
1 Image citation: "Figure 2 from Nomori H, Yue C, Iguchi H, Kashihara K, Wada R, Saito T. Long-term outcomes of combination therapy with stereotactic body radiation therapy plus cryoablation using liquid nitrogen for stage I non-small cell lung cancer with tumors ≥ 2 cm. PLOS ONE. 2025; 5:1–12. https://doi.org/10.1371/journal.pone.0332893, distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0)"
About ProSense®
ProSense® is a minimally invasive cryosurgical tool that provides the option to destroy tumors by freezing them. The system uniquely harnesses the power of liquid nitrogen to create large lethal zones for maximum efficacy in tumor destruction in benign and cancerous lesions, including in the breast, kidney, lung, and liver.
The ProSense® Cryoablation System is the first and only medical device to receive FDA marketing authorization for the local treatment of early-stage, low-risk breast cancer with adjuvant endocrine therapy for women aged 70 and above, including patients who are not suitable for surgical alternatives for breast cancer treatment. A full list of benefits and risks can be found on our website.
ProSense® enhances patient and provider value by accelerating recovery, reducing pain, surgical risks, and complications. With its easy, transportable design and liquid nitrogen utilization, ProSense® opens the door to fast and convenient office-based procedures for breast tumors.
About IceCure Medical
IceCure Medical (NASDAQ:ICCM) develops and markets advanced liquid-nitrogen-based cryoablation therapy systems for the destruction of tumors (benign and cancerous) by freezing, with the primary focus areas being breast, kidney, bone and lung cancer. Its minimally invasive technology is a safe and effective option to surgical tumor removal that is easily performed in a relatively short procedure. The Company's flagship ProSense® system is marketed and sold worldwide for the indications cleared and approved to date including in the U.S., Europe and Asia.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 and other Federal securities laws. Words such as "expects," "anticipates," "intends," "plans," "believes," "seeks," "estimates" and similar expressions or variations of such words are intended to identify forward-looking statements. For example, IceCure is using forward looking statements in this press release when it discusses the potential of its Cryoablation System to improve outcomes for lung cancer patients and its impact in key markets including the U.S. and Europe. Historical results of scientific research and clinical and preclinical trials do not guarantee that the conclusions of future research or trials will suggest identical or even similar conclusions. Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among others: the Company's planned level of revenues and capital expenditures; the Company's available cash and its ability to obtain additional funding; the Company's ability to market and sell its products; legal and regulatory developments in the United States and other countries; the Company's ability to maintain its relationships with suppliers, distributors and other partners; the Company's ability to maintain or protect the validity of its patents and other intellectual property; the Company's ability to expose and educate medical professionals about its products; political, economic and military instability in the Middle East, specifically in Israel; as well as those factors set forth in the Risk Factors section of the Company's Annual Report on Form 20-F for the year ended December 31, 2024 filed with the SEC on March 27, 2025, and other documents filed with or furnished to the SEC which are available on the SEC's website, www.sec.gov. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
IR Contact:
Email: [email protected]
Michael Polyviou
Phone: 732-232-6914
Photo: https://mma.prnewswire.com/media/2811717/ICECURE_Cryoblation.jpg
Logo: https://mma.prnewswire.com/media/2319310/IceCure_Medical_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/icecures-cryoablation-system-combined-with-radiation-therapy-successfully-treats-non-small-cell-lung-cancer-nsclc-with-92-disease-specific-5-year-survival-302602561.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/icecures-cryoablation-system-combined-with-radiation-therapy-successfully-treats-non-small-cell-lung-cancer-nsclc-with-92-disease-specific-5-year-survival-302602561.html
SOURCE IceCure Medical