Iveric Bio Announces New Functional Vision Loss Reduction Data from Avacincaptad Pegol GATHER Trials Presented at ARVO Annual Meeting
- Post-hoc analysis from the GATHER trials, for the first time, signals that reduced rate of vision loss in patients receiving avacincaptad pegol (ACP) was correlated with reduced geographic atrophy (GA) growth -
- Post-hoc time-to-event analyses signal fewer patients receiving ACP had 10-, 15- and 20-letter losses from baseline at two consecutive visits up to 12 months compared to sham -
IVERIC bio, Inc. (NASDAQ:ISEE) today announced new findings from exploratory analyses of data for avacincaptad pegol (ACP), which were presented today at the 2023 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), in New Orleans.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230423005028/en/
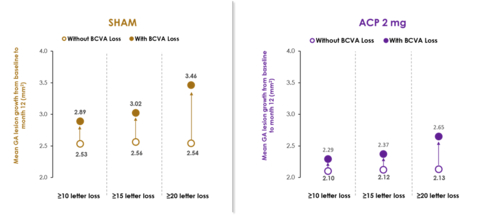
ACP, avacincaptad pegol; BCVA, best corrected visual acuity; GA, geographic atrophy. Without BCVA (vision) loss group was defined as patients who had a change in BCVA from baseline at Month 12 less than the categorical value. (Graphic: Business Wire)
A post-hoc analysis from the GATHER1 and GATHER2 pivotal Phase 3 clinical trials showed, for the first time in an interventional study in GA, a relationship between GA growth and worsening vision loss. In this combined analysis, greater vision loss was correlated with increased GA growth. See accompanying graph.
"This is the first time a relationship between disease progression and worsening visual acuity has been observed in GA, connecting anatomy and function," said Carl Danzig, M.D., Director, Vitreo-Retinal Services, Rand Eye Institute, Deerfield Beach, Florida, who presented the findings at ARVO. "These data suggest that in the ACP-treated group, the reduction in growth of GA resulted in an overall lower rate of vision loss."
As previously announced, the post-hoc analysis of GATHER1 and GATHER2 combined data signaled a 56% risk reduction in the rate of persistent vision loss in GA patients receiving ACP 2 mg compared to sham over the first 12 months of treatment. Persistent vision loss was defined as a loss of ≥15 letters in Best Corrected Visual Acuity (BCVA) from baseline measured at any two consecutive visits up to month 12. Multiple sensitivity analyses were conducted to evaluate this finding, including 10- and 20-letter loss at two consecutive visits up to month 12, and results were consistent.
About Avacincaptad Pegol
Avacincaptad pegol (ACP) is an investigational drug for treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD) that is currently under evaluation for safety and efficacy by the U.S. FDA. ACP is a novel complement C5 protein inhibitor. Overactivity of the complement system and the C5 protein are suspected to play a critical role in the development and growth of scarring and vision loss associated with GA secondary to AMD. By targeting C5, ACP has the potential to decrease activity of the complement system that causes the degeneration of retinal cells and potentially slow the progression of GA.
About Geographic Atrophy
Age-related macular degeneration (AMD) is the major cause of moderate and severe loss of central vision in aging adults, affecting both eyes in the majority of patients. The macula is a small area in the central portion of the retina responsible for central vision. As AMD progresses, the loss of retinal cells and the underlying blood vessels in the macula results in marked thinning and/or atrophy of retinal tissue. Geographic atrophy, associated with AMD, leads to further irreversible loss of vision in these patients.
About the GATHER Clinical Trials
ACP met its primary endpoint in the completed GATHER1 clinical trial and the ongoing GATHER2 clinical trial, both of which are randomized, double-masked, sham-controlled, multicenter Phase 3 clinical trials. These clinical trials evaluated the safety and efficacy of monthly 2 mg intravitreal administration of ACP in patients with GA secondary to AMD. For the first 12 months in both trials, patients were randomized to receive either ACP or sham monthly. There were 286 participants enrolled in GATHER1 and 448 participants enrolled in GATHER2. The primary efficacy endpoints in both pivotal trials were based on GA area measured by fundus autofluorescence at three time points: Baseline, Month 6, and Month 12. The mean rate of growth (slope) in GA area from baseline to month 12 using observed data was 35% in GATHER1 and 18% in GATHER2. In GATHER1 and GATHER2 combined, the most frequently reported treatment emergent adverse events at 12 months in the 2 mg recommended dose were related to the injection procedure. The most common adverse reactions (≥ 5% and greater than sham) reported at 12 months in patients who received ACP 2 mg were conjunctival hemorrhage (13%), increased IOP (9%), and CNV (7%).
About Iveric Bio
Iveric Bio is a science-driven biopharmaceutical company focused on the discovery and development of novel treatments for retinal diseases with significant unmet medical needs. The Company is committed to having a positive impact on patients' lives by delivering high-quality, safe, and effective treatments designed to address debilitating retinal diseases including earlier stages of age-related macular degeneration. For more information on the Company, please visit www.ivericbio.com.
Forward-looking Statements
Any statements in this press release about the Company's future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include any statements about the Company's strategy, future operations and future expectations and plans and prospects for the Company, and any other statements containing the words "anticipate," "believe," "estimate," "expect," "intend", "goal," "may", "might," "plan," "predict," "project," "seek," "target," "potential," "will," "would," "could," "should," "continue," "signal," and similar expressions. In this press release, the Company's forward-looking statements include statements about its expectations regarding the results and implications of the clinical data from its GATHER1 and GATHER2 trials of ACP in geographic atrophy, including the relevance of post-hoc analyses from these trials, and the potential safety and efficacy of ACP in treating GA. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company's development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to expectations for regulatory matters, interpretation of clinical trial results by the scientific and medical community, developments from the Company's competitors and the marketplace for the Company's products, and other factors discussed in the "Risk Factors" section contained in the quarterly and annual reports that the Company files with the Securities and Exchange Commission. Any forward-looking statements represent the Company's views only as of the date of this press release. The Company anticipates that subsequent events and developments may cause its views to change. While the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230423005028/en/
