MannKind Announces Six-Month Results From Phase 3 INHALE-1 Pediatric Diabetes Trial Utilizing Inhaled Insulin (Afrezza®)
- Company plans to meet with FDA regarding potential sNDA submission in 1H 2025
- Call planned today at 8:30 a.m. (ET) to discuss company's diabetes program progression
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., Dec. 16, 2024 (GLOBE NEWSWIRE) -- MannKind Corporation (NASDAQ:MNKD), a company focused on the development and commercialization of inhaled therapeutic products and devices for patients with endocrine and orphan lung diseases, today announced six-month results from its Phase 3 INHALE-1 study of Afrezza (insulin human) Inhalation Powder in children and adolescents (aged 4-17 years of age). MannKind expects to submit a request for a supplemental new drug application (sNDA) meeting with the U.S. Food and Drug Administration (FDA) in 1H 2025 to discuss the data and filing timeline.
The INHALE-1 study is a 26-week, open-label clinical trial that randomized 230 subjects to one of two groups: Afrezza or multiple daily injections (MDI) of rapid acting insulin analog (RAA) in combination with basal insulin. The primary endpoint was a non-inferior change in HbA1c levels after 26 weeks. A 26-week extension phase in which all remaining MDI patients switched to Afrezza is still ongoing.
Results were as follows:
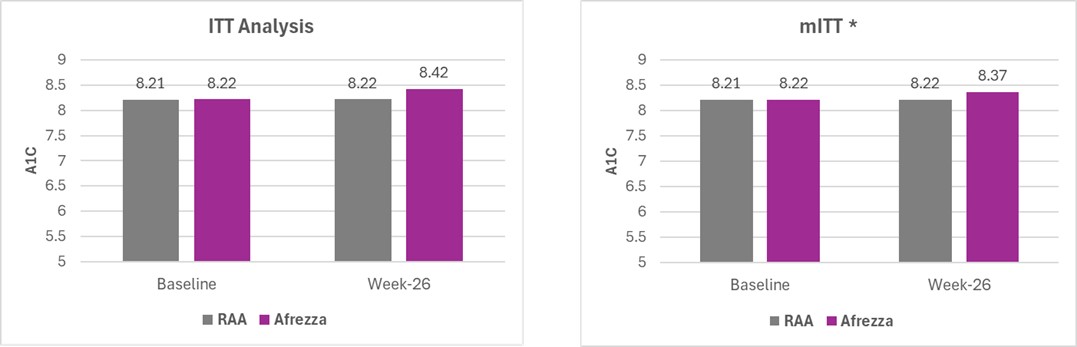
* mITT analysis excludes one outlier from the primary ITT endpoint who failed to adhere to the study protocol
An analysis of the full intent-to-treat population (ITT) found that the between-group difference in mean HbA1c change over 26 weeks exceeded the prespecified non-inferiority margin of 0.4% (0.435%), largely driven by the variability of a single patient who did not adhere to the study protocol. A modified ITT (mITT) analysis, which excluded this subject, did not exceed the predetermined threshold of 0.4% (0.370%), thereby establishing the non-inferiority of Afrezza to MDI, which was the primary endpoint of the study.
Over 26 weeks of treatment, no difference in lung function parameters were seen between the treatment groups. The Afrezza-treated patients had a mean FEV1 of 2.901 liters (99.6% of predicted) at baseline and 2.934 liters (96.6% of predicted) at 26 weeks. MDI-treated patients had corresponding mean FEV1 values of 2.948 liters (102.3% of predicted) and 2.957 liters (98% of predicted), respectively. Additional safety findings, including for hypoglycemia, did not show any significant concerns or differences between the treatment groups.
"The overall efficacy and safety outcomes seen in the first 26 weeks are encouraging. This represents a monumental step in our more than 25-year history of pioneering the development of inhaled insulin and working to bring this new treatment option to children and adolescents over the past seven years," said Dr. Kevin Kaiserman, Senior Vice President, Therapeutic Area Head, Endocrine Diseases for MannKind Corporation.
"It was exciting to partner with MannKind and help lead this study to potentially expand the use of inhaled insulin, which is currently used successfully by many adults with diabetes, to a population that hasn't had a treatment option other than injectable insulin in the history of their care," said Dr. Roy W. Beck, founder of the Jaeb Center for Health Research who provided oversight for INHALE-1. "The six-month results are clinically meaningful and show Afrezza as a potential future treatment option for a growing pediatric population living with type 1 and type 2 diabetes."
Conference Call
MannKind will host a live audio webcast beginning at 8:30 a.m. Eastern Time on Monday, December 16, 2024, to share results and discuss the company's diabetes program progression. Participating in the conference call from MannKind will be Chief Executive Officer Michael Castagna, PharmD and Dr. Kaiserman. The webcast will be accessible via a link on MannKind's website at https://investors.mannkindcorp.com/events-and-presentations. A replay will also be available in the same location within 24 hours following the call and be accessible for approximately 90 days.
About Afrezza
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to improve glycemic control in adults with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis or in patients that smoke or have recently stopped smoking.
Important Safety Information
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and COPD
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.
Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
Please see additional Important Safety Information, Full Prescribing Information, including BOXED WARNING, available on Afrezza.com/safety.
About MannKind
MannKind Corporation (NASDAQ:MNKD) focuses on the development and commercialization of innovative inhaled therapeutic products and devices to address serious unmet medical needs for those living with endocrine and orphan lung diseases.
We are committed to using our formulation capabilities and device engineering prowess to lessen the burden of diseases such as diabetes, nontuberculous mycobacterial (NTM) lung disease, pulmonary fibrosis, and pulmonary hypertension. Our signature technologies – dry-powder formulations and inhalation devices – offer rapid and convenient delivery of medicines to the deep lung where they can exert an effect locally or enter the systemic circulation, depending on the target indication.
With a passionate team of Mannitarians collaborating nationwide, we are on a mission to give people control of their health and the freedom to live life.
Please visit mannkindcorp.com to learn more, and follow us on LinkedIn, Facebook, X or Instagram.
Forward-Looking Statements
This press release contains forward-looking statements about a planned meeting with the FDA, a potential sNDA submission and the potential expanded use of Afrezza that involves risks and uncertainties. Words such as "believes", "anticipates", "plans", "expects", "intends", "will", "goal", "potential" and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon MannKind's current expectations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that issues that develop in the review by the FDA may subject us to unanticipated delays or prevent us from obtaining the expanded indication as well as other risks detailed in MannKind's filings with the Securities and Exchange Commission, including under the "Risk Factors" heading of its Annual Report on Form 10-K for the year ended December 31, 2023, and subsequent periodic reports on Form 10-Q and current reports on Form 8-K. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. All forward-looking statements are qualified in their entirety by this cautionary statement, and MannKind undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date of this press release.
AFREZZA and MANNKIND are registered trademarks of MannKind Corporation.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/12f4dac8-7936-41b3-a2ad-7eef85fe3712

For MannKind: Media Relations Christie Iacangelo, (818) 292-3500 Email: [email protected] Investor Relations Ana Kapor, (415) 377.2882 Email: [email protected]

