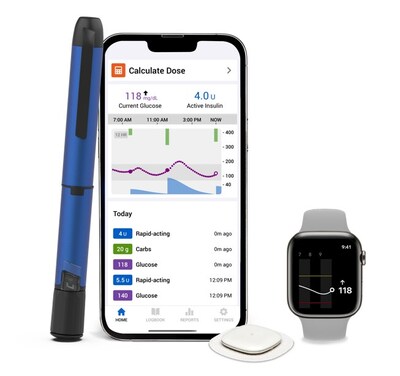
Medtronic receives FDA clearance for new InPen™ app, paving the way for its Smart MDI system launch with Simplera™ CGM
New Smart MDI system will be the first system to deliver real-time, personalized insights on when and how much to dose including for missed or inaccurate mealtime doses.
GALWAY, Ireland, Nov. 20, 2024 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced U.S. Food and Drug Administration (FDA) clearance for its new InPen™ app featuring missed meal dose detection, paving the way for the launch of its Smart MDI system with the Simplera™ continuous glucose monitor (CGM). The company's Smart MDI system combines its InPen™ smart insulin pen with its newest Simplera™ CGM — the company's first disposable, all-in-one CGM that's half the size of previous Medtronic CGMs.
With this clearance, the system will be the first in the market to recommend corrections for missed or inaccurate insulin doses, providing real-time, personalized insights for individuals on multiple daily injection (MDI) therapy.
For people with diabetes who need daily insulin injections, bolusing before a meal is essential as it helps regulate glucose levels and prevent blood sugar spikes after eating. Minimizing the frequency of these glucose highs reduces the risk of both short- and long-term complications and supports better overall health. However, it's estimated that individuals living with diabetes regularly miss 1 out of 3 doses. The Missed Dose alert function helps to minimize the frequency of these glucose highs.1 The Medtronic Smart MDI system reduces the guesswork out of diabetes management, helping to address a significant unmet need for MDI users who struggle with juggling numerous decisions related to insulin dosing on a daily basis.
"I'm thrilled about the launch of the Medtronic Smart MDI system with the InPen™ app and Simplera™ CGM. This is a significant leap forward for those on multiple daily injections, offering intelligent dosing insights and simplifying diabetes management," said Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, FADCES, FCCP. "By reducing the guesswork out of insulin dosing, this tool helps maintain stable blood sugars, optimize long-term health, and reduce complications from hyperglycemia."2,3
Medtronic will initiate a limited market release beginning with existing standalone CGM and InPen™ customers followed by a broad commercial launch.
1. MacLeod, J, Heungyong Im, G, Smith, M, Vigersky, RA. Shining the Spotlight on Multiple Daily Insulin Therapy: Real-World Evidence of the InPen Smart Insulin Pen. Diabetes Technology & Therapeutics. 2024. 26:1, 33-39. |
2. Vigersky et al., Impact Of InPen Smart Insulin Pen Use on Real-World Glycemic and Insulin Dosing Outcomes in Individuals with Poorly Controlled Diabetes. Presented at: American Diabetes Association; 81st Scientific Sessions; 2021 Jun 25-29. |
3. Chien A, Thanasekaran S, Gaetano A, Im G, Wherry K, MacLeod J, Vigersky RA. Potential cost savings in the United States from a reduction in sensor-detected severe hypoglycemia among users of the InPen smart insulin pen system. J Manag Care Spec Pharm. 2023 Mar;29(3):285-292. |
About the Diabetes Business at Medtronic (www.medtronicdiabetes.com)
Medtronic Diabetes is on a mission to alleviate the burden of diabetes by empowering individuals to live life on their terms, with the most advanced diabetes technology and always-on support when and how they need it. We've pioneered first-of-its-kind innovations for over 40 years and are committed to designing the future of diabetes management through next-generation sensors (CGM), intelligent dosing systems, and the power of data science and AI while always putting the customer experience at the forefront.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Galway, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow Medtronic on LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Contacts: | ||
Janet Cho | Ryan Weispfenning | |
Public Relations | Investor Relations | |
+1-818-403-7028 | +1-763-505-4626 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-receives-fda-clearance-for-new-inpen-app-paving-the-way-for-its-smart-mdi-system-launch-with-simplera-cgm-302310779.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-receives-fda-clearance-for-new-inpen-app-paving-the-way-for-its-smart-mdi-system-launch-with-simplera-cgm-302310779.html
SOURCE Medtronic plc