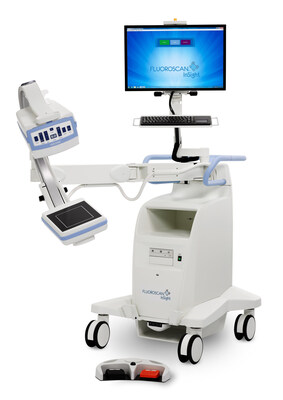
Minicarm.com Named Exclusive U.S. Distributor of Hologic's Fluoroscan® Insight FD Mini C-Arm
HAUPPAUGE, N.Y., Sept. 29, 2025 /PRNewswire/ -- Minicarm.com, a leading provider of imaging solutions for orthopedic and surgical practices, is proud to announce that it has secured an allocation of the Hologic Fluoroscan® Insight™ FD Mini C-Arm for resale in the United States. As Hologic transitions away from commercial activities related to the Fluoroscan Insight product line, Minicarm.com will continue to offer sales support for the remaining inventory, ensuring continuity and specialized service for U.S. customers.
With this acquisition, Minicarm.com is uniquely positioned to offer U.S. customers who are seeking to purchase Hologic's market-leading Fluoroscan Insight FD system access to a select inventory, ensuring streamlined access, dedicated customer support, and comprehensive service.
The Fluoroscan Insight FD is a compact, versatile imaging system designed to deliver high-quality, real-time fluoroscopic imaging for orthopedic and extremity procedures. Renowned for its exceptional image clarity, user-friendly design, and versatility, the Insight FD system enables physicians to make accurate clinical decisions while enhancing workflow efficiency.
"We are thrilled to work closely with Hologic to make sure their Fluoroscan Insight FD systems are available to US customers at this pivotal time," said Joshua Bacon, VP, Minicarm.com. "This collaboration allows us to bring one of the most trusted and advanced Mini C-Arm systems to orthopedic practices, hospitals, and surgical centers nationwide."
As part of the distribution agreement, Minicarm.com will:
- Provide direct sales, marketing, and customer support across the U.S.
- Offer comprehensive training and education for physicians and staff.
Customers looking to upgrade their Hologic Fluoroscan Insight 2 or older Hologic Fluoroscan Insight FD Mini C-Arm systems, or those seeking more details about the new Hologic Fluoroscan Insight FD Mini C-Arm, can reach out to Minicarm.com. Call us at (800) 643-2998, email [email protected], or visit www.minicarm.com for more information.
About Minicarm.com
Minicarm.com is a leading distributor and service provider of advanced Mini C-Arm imaging solutions, founded by Christopher Bacon, the technical founder of Orthoscan. With deep product expertise, a strong commitment to customer service, and nationwide support, Minicarm.com is dedicated to expanding access to high-quality imaging technology for healthcare providers worldwide.
About Hologic
Hologic, Inc. (NASDAQ: HOLX) is an innovative medical technology company primarily focused on improving women's health and well-being through early detection and treatment. Its products include industry-leading imaging, diagnostic, and surgical solutions.
Media Contact:
Joshua Bacon
Minicarm.com
Phone: (800) 643-2998
Email: [email protected]
Website: www.minicarm.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/minicarmcom-named-exclusive-us-distributor-of-hologics-fluoroscan-insight-fd-mini-c-arm-302567568.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/minicarmcom-named-exclusive-us-distributor-of-hologics-fluoroscan-insight-fd-mini-c-arm-302567568.html
SOURCE Minicarm.com