Preliminary Phase 1b/2 Data for REC-4881 in Familial Adenomatous Polyposis (FAP) Demonstrates Reduced Polyp Burden
- In the Phase 2 open-label study, REC-4881 (4 mg QD) led to a median 43% reduction (n=6 patients) in polyp burden at the Week 13 assessment at time of data cutoff.
- Five of six patients (83%) experienced reductions in polyp burden ranging from 31% to 82%, however, one patient showed a substantial increase from baseline.
- At Week 13, 50% of patients (3 out of 6) achieved ≥1-point improvement in Spigelman stage, a measure of upper GI disease severity.
- The early safety profile of REC-4881 was generally consistent with that of prior MEK1/2 inhibitors; among 19 patients across Phase 1b and 2, most treatment-related adverse events were Grade 1 or 2, with Grade 3 events in 16% of patients and no Grade ≥4 TRAEs reported to date.
SALT LAKE CITY, May 04, 2025 (GLOBE NEWSWIRE) -- Recursion (NASDAQ:RXRX), a clinical-stage TechBio company decoding biology to radically improve lives, today announced preliminary safety and efficacy results from its ongoing Phase 1b/2 TUPELO trial of REC-4881, an investigational, allosteric MEK1/2 inhibitor in development for Familial Adenomatous Polyposis (FAP). These data were presented in a late-breaking oral presentation at Digestive Disease Week (DDW) 2025 in San Diego, California.
FAP is a rare, inherited disorder caused by mutations in the APC gene, leading to the growth of hundreds to thousands of gastrointestinal polyps and a near 100% lifetime risk of colorectal cancer if left untreated. Despite this significant burden, there are currently no FDA-approved treatments. FAP affects an estimated 50,000 individuals across the US and EU5 (France, Germany, Italy, Spain, and the UK). REC-4881 has received Fast Track and Orphan Drug designations from the U.S. FDA, as well as Orphan Drug designation from the European Commission.
As of the March 17, 2025 data cutoff in the open-label Phase 2 portion of the TUPELO trial, treatment with REC-4881 (4 mg QD) led to a preliminary median 43% reduction to date in polyp burden at the Week 13 assessment among six efficacy-evaluable patients. Other investigational agents in separate studies have reported 20-30% polyp burden reduction in 6 months1. Five of the six patients (83%) experienced reductions in polyp burden ranging from 31% to 82%, while one patient (17%) showed a substantial increase of 595% from baseline. Additionally, three of six patients (50%) achieved a ≥1-point reduction in Spigelman stage; one patient had no change, one progressed from Stage II to IV, and one had no baseline stage but was classified as Stage II at Week 13.
Across the combined Phase 1b and ongoing Phase 2 cohorts (n=19 safety-evaluable patients), REC-4881 demonstrated an early safety profile generally consistent with that of prior MEK1/2 inhibitors. Seventy-nine percent (15 of 19) of patients experienced at least one treatment-related adverse event (TRAE), the majority of which were Grade 1 or 2 in severity. Grade 3 TRAEs occurred in 16% of patients, with no Grade ≥4 events reported to date. In Phase 1b, the most frequent TRAEs (≥20%) were acneiform rash, diarrhea, and decreased left ventricular ejection fraction (LVEF), while in Phase 2, the most common events were acneiform rash, blood creatine phosphokinase (CPK) increase, and diarrhea. Overall, treatment modifications were infrequent, with 3 of 19 patients discontinuing treatment, 1 patient each requiring dose interruption or modification.
"For patients with FAP, who face increased risk of colorectal and small bowel cancer and a lifetime of invasive interventions, the preliminary polyp burden reduction at a median of >43% seen with REC-4881 1in just three months of treatment is highly encouraging," said Dr. Jewel Samadder, Gastroenterologist at Mayo Clinic and Principal Investigator of TUPELO, "These early results offer a much-needed glimpse of hope for this underserved population."
"For patients with FAP, who currently lack FDA-approved treatment options, Recursion's AI-powered Recursion OS platform identified a promising approach through MEK 1/2 inhibition," said Najat Khan, PhD, Chief R&D Officer and Chief Commercial Officer at Recursion. "By analyzing cellular models of APC gene loss, we uncovered a potential first-in-disease treatment and are excited to share our preliminary findings."
About the TUPELO Trial Design (REC-4881)
The Phase 1b/2 TUPELO trial is evaluating the safety, tolerability, pharmacokinetics (PK), and preliminary efficacy of REC-4881 monotherapy in patients with Familial Adenomatous Polyposis (FAP).
The Phase 1b portion was a randomized, double-blind, placebo-controlled safety run-in designed to assess the safety, tolerability, and PK of REC-4881 at 4 mg QD for 14 days in FAP patients aged 18 years and older. A total of five patients received REC-4881, and two received placebo. Following the safety review, the eligibility criteria were amended to enroll only patients aged ≥55 years in Phase 2, in an effort to reduce treatment-related adverse events (TRAEs) consistent with MEK1/2 inhibition.
In the ongoing Phase 2 portion, participants must have a confirmed germline APC mutation and be ≥55 years old. Efficacy is assessed via upper and lower endoscopy at baseline, Week 13 (W13, on-treatment), and Week 25 (W25, off-treatment). The primary efficacy endpoint is percent change from baseline in polyp burden.
The Efficacy Evaluable Population includes patients who had measurable disease at baseline, received ≥75% of the study drug, and completed at least one post-baseline endoscopic assessment. Disease staging is evaluated using the Spigelman system for the upper gastrointestinal tract and the InSiGHT classification for the lower GI tract.
Efficacy
In the interim Phase 2 analysis, REC-4881 (4 mg QD) demonstrated a preliminary median 43% reduction in polyp burden at Week 13 among efficacy-evaluable post-colectomy FAP patients aged ≥55 years (n=6). Other investigational agents in separate studies have reported 20-30% polyp burden reduction in 6 months1. Three of six patients (50%) achieved a >50% reduction in polyp burden at the Week 13 (on-treatment) assessment. Of these, two patients maintained a >30% reduction at the Week 25 (off-treatment) assessment, 12 weeks after completing therapy. One patient who reached Week 25 did not undergo the endoscopic evaluation. The distribution of polyp burden changes across all patients is shown in Figure 1.
At Week 13, three of six patients achieved a ≥1-point reduction in Spigelman stage, a validated measure of upper gastrointestinal disease severity; one patient had no change, one progressed from Stage II to IV, and one had no baseline stage but was classified as Stage II at Week 13.
At the time of data cutoff, three additional patients had been enrolled in the 8 mg QD dose cohort. All three were found to have no measurable disease (zero polyp burden) at baseline endoscopy and were therefore not considered efficacy evaluable.
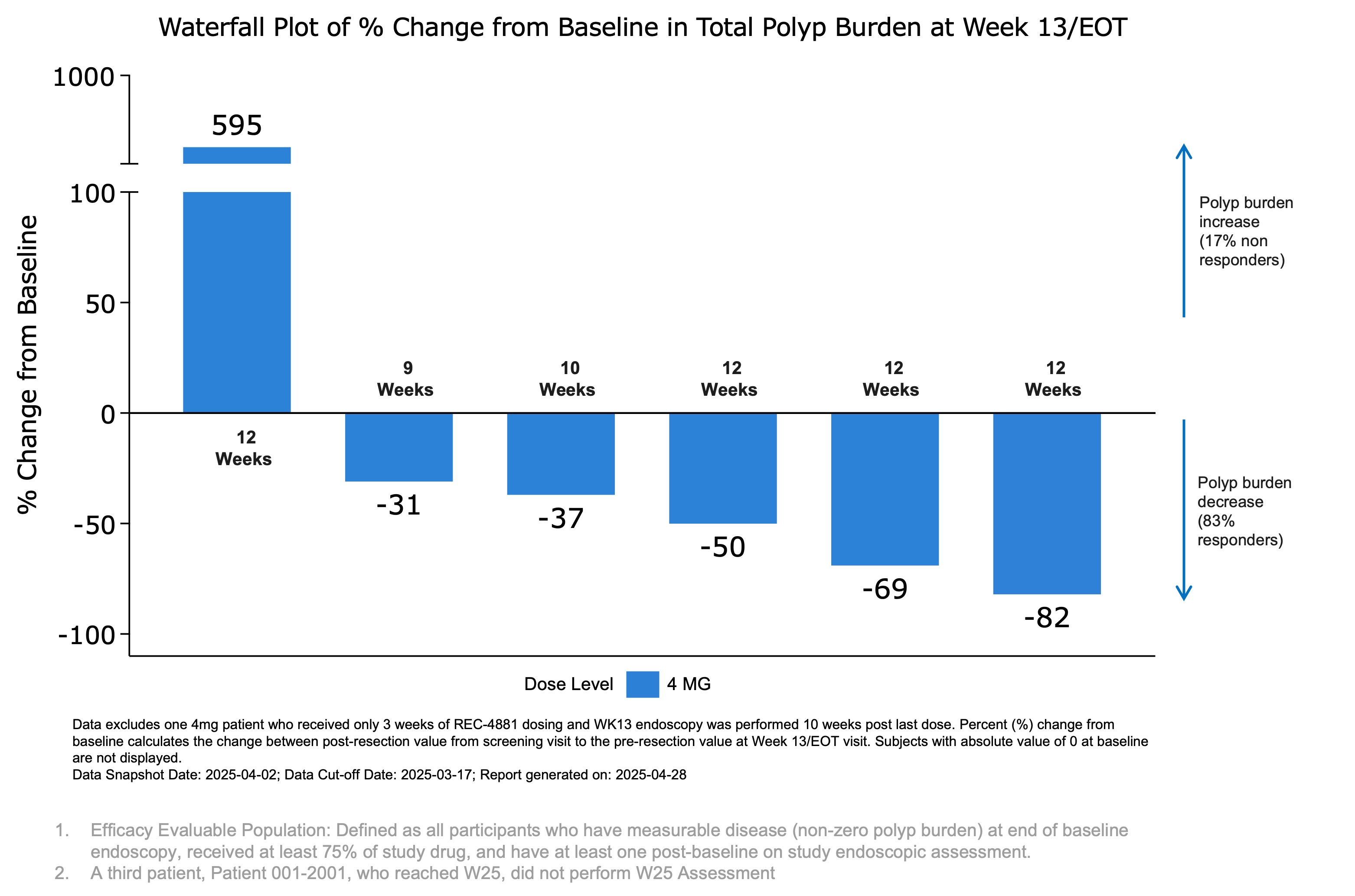
Figure 1: Waterfall plot showing percent change from baseline in total polyp burden at Week 13 (end of treatment) for efficacy-evaluable patients receiving REC-4881 (4 mg QD).
Safety
In the preliminary Phase 1b safety and Phase 2 dose-finding/dose-expansion cohorts of the TUPELO trial, the majority of treatment-related adverse events (TRAEs) were Grade 1 or 2, with Grade 3 TRAEs observed in 16% of patients and no Grade ≥4 TRAEs reported to date. Among the 19 safety-evaluable patients that received REC-4881 across both phases, the most frequent TRAEs (≥20%) in Phase 1b were acneiform rash, diarrhea, and decreased left ventricular ejection fraction (LVEF). In the ongoing Phase 2 portion, the most commonly reported TRAEs (≥20%) have been acneiform rash, elevated blood creatine phosphokinase (CPK), and diarrhea. Rash and decreased left ventricular ejection fraction (LVEF), both observed in this study, are consistent with the reported safety profile of MEK1/2 inhibitors.
Grade 3 TRAEs included rash, increased C-reactive protein (CRP), and decreased LVEF—each occurring in one patient (6%), all within the 4 mg dose cohort. A single patient in the Phase 1b cohort experienced a Grade 3 LVEF decrease, which was transient and resolved following dose interruption. No patients in Phase 1b discontinued treatment due to a TRAE. Three patients in Phase 2 discontinued treatment due to TRAEs. A full summary of adverse events is provided in Figure 2.
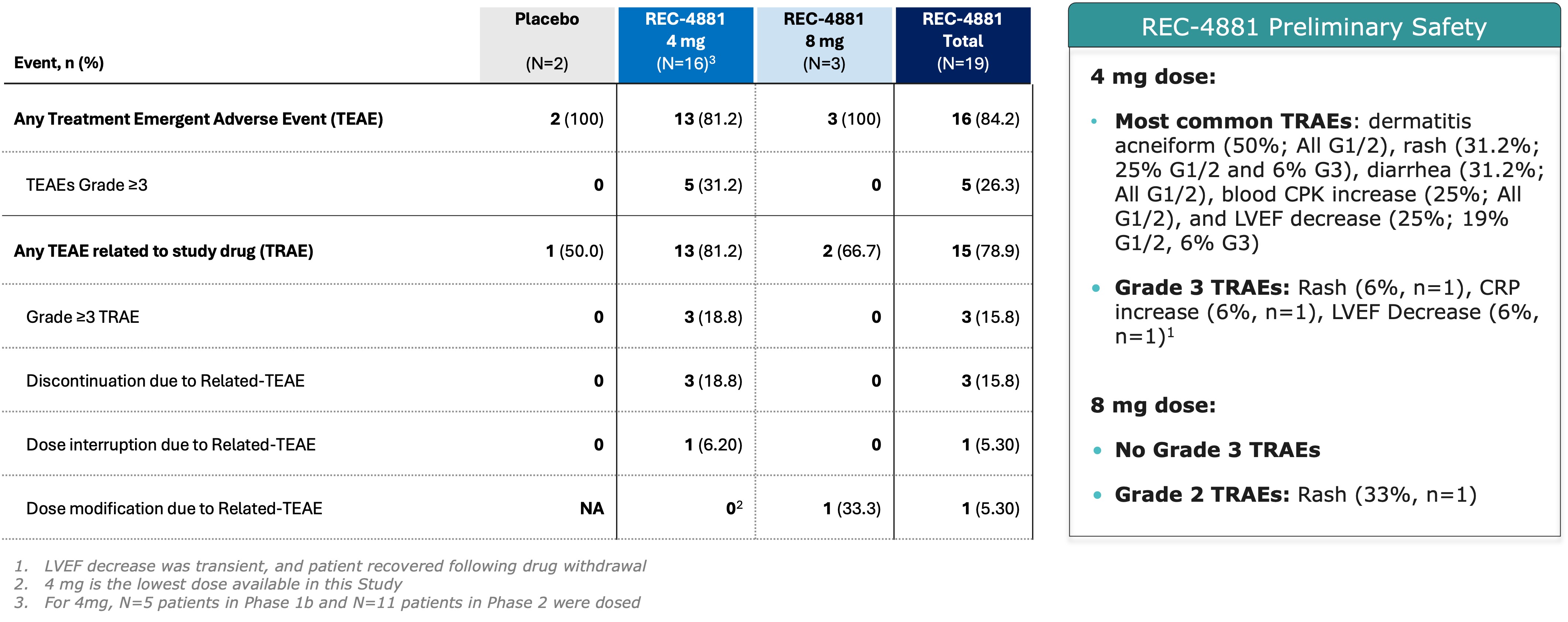
Figure 2. Summary of Adverse Events Across Phase 1b and Phase 2 of the TUPELO Trial
Next Steps
Patient enrollment in the TUPELO trial is ongoing. Recursion plans to conduct additional efficacy and safety analyses anticipated in the second half of 2025.
About REC-4881
REC-4881 is an orally bioavailable, non-ATP-competitive allosteric small molecule MEK 1/2 inhibitor. The platform analyzed cellular models of APC gene loss—the root cause of FAP—to identify compounds that suppress the disease-inducing effects of APC mutations.
About Recursion
Recursion (NASDAQ:RXRX) is a clinical stage TechBio company leading the space by decoding biology to radically improve lives. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously generate one of the world's largest proprietary biological and chemical datasets. Recursion leverages sophisticated machine-learning algorithms to distill from its dataset a collection of trillions of searchable relationships across biology and chemistry unconstrained by human bias. By commanding massive experimental scale — up to millions of wet lab experiments weekly — and massive computational scale — owning and operating one of the most powerful supercomputers in the world, Recursion is uniting technology, biology and chemistry to advance the future of medicine. Recursion is headquartered in Salt Lake City, where it is a founding member of BioHive, the Utah life sciences industry collective. Recursion also has offices in Toronto, Montréal, New York, London, Oxford area, and the San Francisco Bay area. Learn more at www.Recursion.com, or connect on X (formerly Twitter) and LinkedIn.
Forward-Looking Statements
This document contains information that includes or is based upon "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995, including, without limitation, those regarding the potential efficacy of REC-4881, including the potential to be a first-in-disease treatment; the timing of and outcomes of the TUPELO clinical trial; the effects of our platform on trial outcomes; the potential size of the market opportunity for our drug candidates; Recursion's leadership of the TechBio space; the Recursion OS and other technologies; and all other statements that are not historical facts. Forward-looking statements may or may not include identifying words such as "plan," "will," "expect," "anticipate," "intend," "believe," "potential," "continue," and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties such as those described under the heading "Risk Factors" in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K for the Fiscal Year Ended December 31, 2024. All forward-looking statements are based on management's current estimates, projections, and assumptions, and Recursion undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.
Media Contact
[email protected]
Investor Contact
[email protected]
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/d025ef61-3451-47e1-9dc6-8fe910b4ef6d
https://www.globenewswire.com/NewsRoom/AttachmentNg/b64bdf18-73b8-4f07-b38d-a96478162b1b
1 Steinberg et al 2000 NEJM and Burke et al 2024 Gastroenterology

