Recursion and Exscientia, two leaders in the AI drug discovery space, have officially combined to advance the industrialization of drug discovery
- Recursion unveils post-combination technology-enabled portfolio with more than 10 clinical and preclinical programs, 10 advanced discovery programs, and more than 10 partnered programs
- Platform will focus on first and best-in-class drug discovery and development, demonstrating the ability to find novel insights and dramatically reduce the time and cost of discovery
- Recursion will host an update call today, November 20, 2024 at 7:30 a.m. ET / 5:30 a.m. MT / 12:30 p.m. GMT on LinkedIn, X and Youtube
SALT LAKE CITY, Nov. 20, 2024 (GLOBE NEWSWIRE) -- The business combination of two AI-powered drug discovery and development companies, Recursion (NASDAQ:RXRX) and Exscientia has been completed, with Exscientia becoming a wholly owned subsidiary of Recursion creating a vertically-integrated and technology-enabled drug discovery platform. Exscientia ADSs (NASDAQ:EXAI) ceased trading and will be delisted from Nasdaq.
"I believe the combination of the incredible teams and platforms at Exscientia and Recursion position us as the leader of the AI-enabled drug discovery and development space," said Chris Gibson, Ph.D., Co-Founder and CEO of Recursion. "With more than 10 clinical and preclinical programs in the internal pipeline, more than 10 partnered programs and over $450M in upfront and realized milestone payments received from partners to date out of more than $20B possible, we are advancing a flywheel of discovery and creating value in our pipeline through technology."
"The combination of our platforms and people make us the company to beat," said David Hallett, Ph.D., former CSO and Interim CEO of Exscientia and newly appointed Chief Scientific Officer at Recursion. "With our combined strength of real-world proprietary data and the models we've created – hypothesizing, testing and learning in a continuous loop – we're redefining the space by shrinking timelines and costs, identifying and optimizing lead candidates faster than traditional methods."
The Company is pleased to share updates on the combined entity's pipeline, partnerships, and platform below:
Pipeline
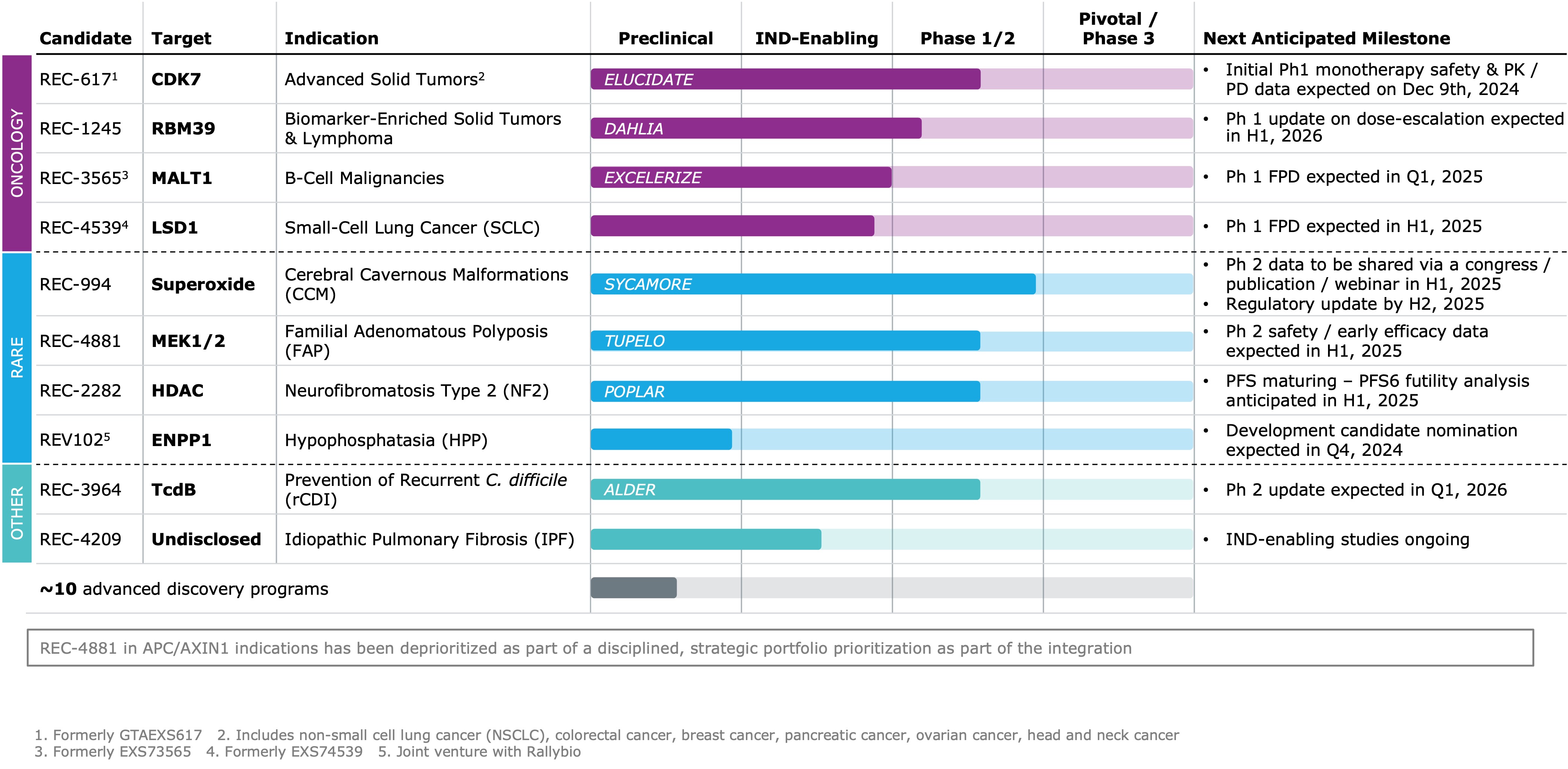
The combined pipeline represents more than 10 clinical and preclinical programs. In addition there are approximately 10 advanced discovery programs in the current pipeline.
Updated guidance is bulleted below as well as a snapshot of our pipeline:
- REC-617 (CDK7 inhibitor; Advanced Solid Tumors): Initial Phase 1 monotherapy safety and PK/PD data expected at the AACR Special Conference on December 9th 2024, and a webinar to follow on December 10th 2024.
- REV102 (ENPP1 inhibitor; Hypophosphatasia): Development candidate nomination expected in Q4 2024
- REC-4881 (MEK1/2 inhibitor, Familial Adenomatous Polyposis): Phase 1b/2 safety and early efficacy data expected in H1 2025
- REC-2282 (pan-HDAC inhibitor; Neurofibromatosis Type 2): PFS6 futility analysis expected by H1 2025
- REC-3565 (MALT1 inhibitor, B-Cell Malignancies): Phase 1 first patient dosed (FPD) expected in Q1 2025
- REC-4539 (LSD1 inhibitor, Small-Cell Lung Cancer): Phase 1 first patient dosed (FPD) expected in H1 2025
- REC-994 (Superoxide scavenger, Cerebral Cavernous Malformation): Further data to be shared at an upcoming medical conference / publication / webinar in H1 2025; regulatory update expected by H2 2025
- REC-394 (C. difficile Toxin B selective inhibitor, C. difficile): Phase 2 update expected in Q1 2026
- REC-1245 (RBM39 degrader; Solid Tumors and Lymphoma): Phase 1 dose-escalation data update expected in H1 2026
- REC-4209 (undisclosed target; Idiopathic Pulmonary Fibrosis): IND-enabling studies are ongoing
- REC-4881 in APC/AXIN1 indications have been deprioritized as part of a disciplined strategic prioritization of the portfolio. Study status will be updated on clinicaltrials.gov
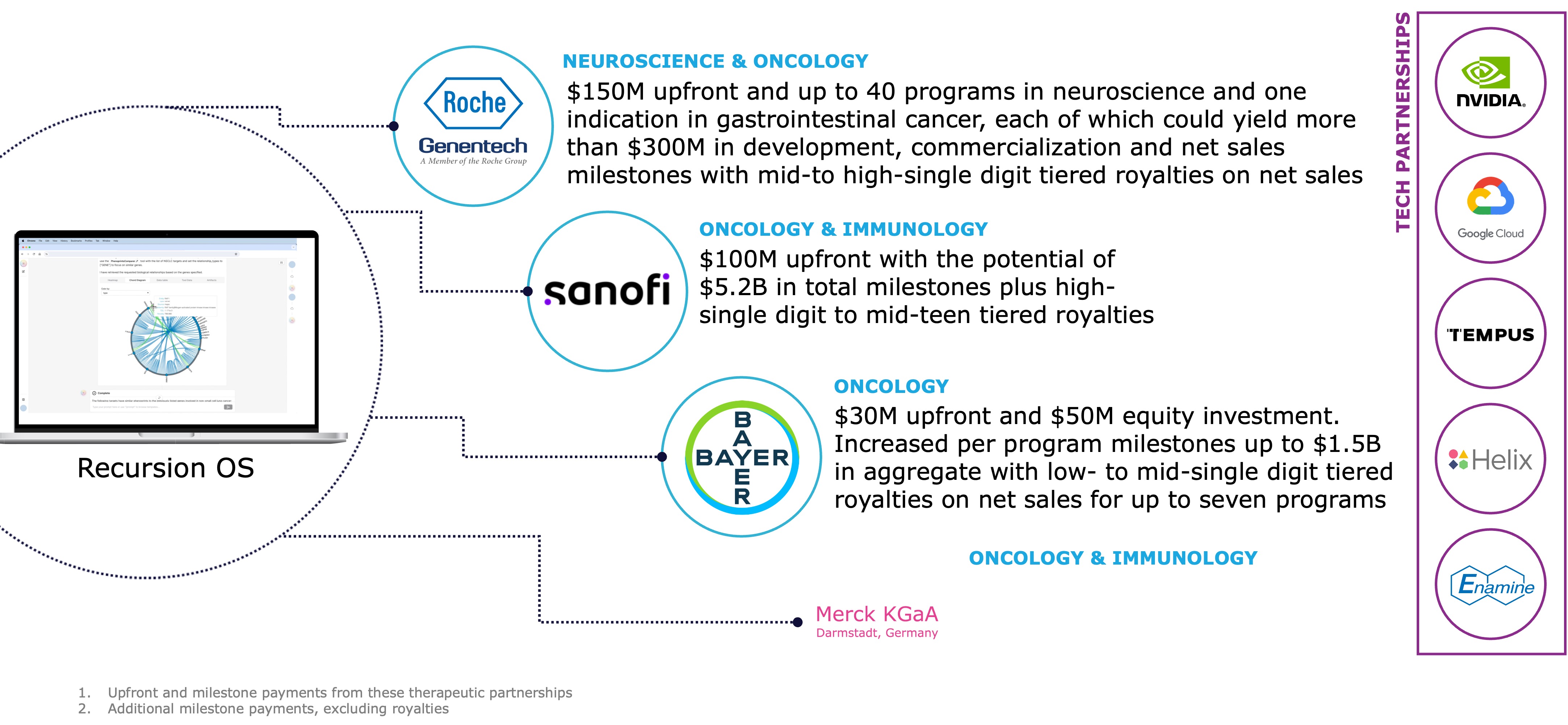
Partnerships
The combined company's therapeutic partnerships represent more than 10 partnered programs in areas such as oncology and immunology. The combined company has received approximately $450M in upfront and milestone payments from partnerships to date. Through these partnerships, we have the potential to receive more than approximately $20B in additional milestone payments before royalties.
Platform
With chemical design and synthesis methods from Exscientia and over 60 petabytes of proprietary data generated in house or licensed from partners like Helix and Tempus, the combined entity will strengthen the Recursion OS to be a first-in-class and best-in-class drug discovery and development platform.
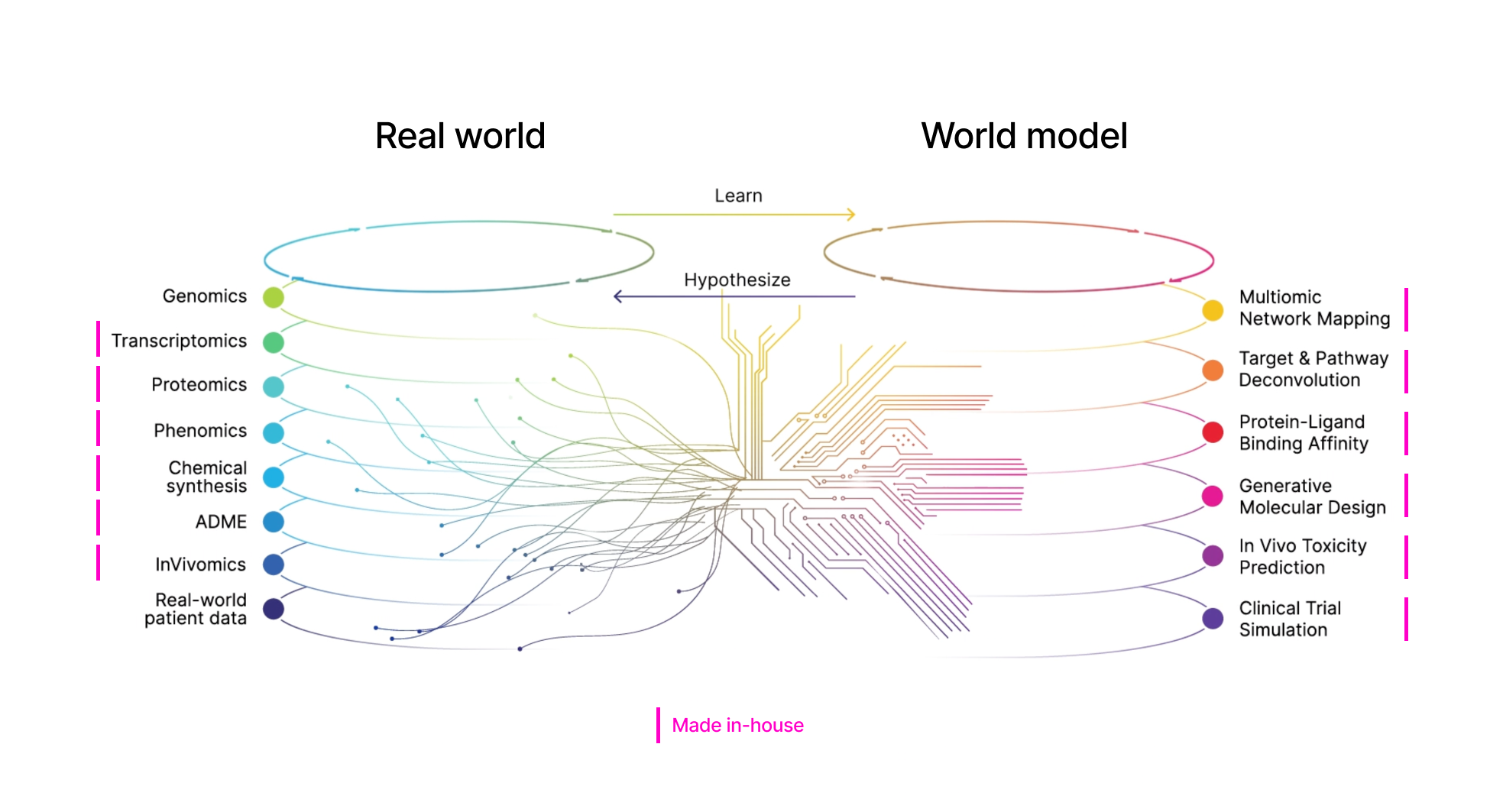
The platform will continue to drive iterative loops of hypotheses and active learning all the way from research to development, with the goal of eventually creating virtual cells that will allow the company to execute clinical trials at scale.
Company, Board, and Leadership Updates
The combined company will have approximately 800 employees with the headquarters remaining in Salt Lake City, and primary offices in Toronto, Montreal, Milpitas, New York, the Oxford area, and London.
Individual board and executive leadership changes of Recursion, effective as of November 20, 2024, are summarized below:
- Franziska Michor, a former member of the Board of Directors of Exscientia, was appointed as a Class II Director of the Board of Directors of Recursion, with her initial term to extend until the 2026 Annual Meeting of Stockholders of Recursion.
- Ben Taylor, former Chief Financial and Strategy Officer of Exscientia, was appointed as the Chief Financial Officer of the Company and President of Recursion UK.
- Dave Hallett, former Interim Chief Executive Officer of Exscientia, was appointed as Chief Scientific Officer of the Company.
- Kristen Rushton, Chief Business Operations Officer of the Company, was promoted to Chief Operating Officer of the Company.
- Matthew Kinn, Senior Vice President, Business Development and Corporate Initiatives of the Company was promoted to serve as Chief Business Officer of the Company.
- Lina Nilsson, Senior Vice President, Emerging Technologies of the Company, was promoted to serve on the executive team as Senior Vice President, Head of Platform of the Company.
- Michael Secora, Tina Marriott, and Laura Schaevitz will transition from their executive roles into advisor roles for the combined company. All three have provided many years of dedicated service to the Company and we wish to express our heartfelt gratitude for each of them. Recursion would not be where it is today without their dedication and efforts.
Update Call Information
Recursion will host an update call today at 7:30 a.m. ET / 5:30 a.m. MT / 12:30 p.m. GMT. The Company will broadcast the live stream from Recursion's X (formerly Twitter), LinkedIn and YouTube accounts, and on Exscientia's LinkedIn account. Questions can be submitted via this link ahead of time or during the livestream.
About Recursion
Recursion is a leading, clinical-stage TechBio company decoding biology to industrialize drug discovery. Central to its mission is the Recursion Operating System (OS), a platform built across diverse technologies that continuously expands one of the world's largest proprietary biological, chemical and patient-centric datasets. Recursion leverages sophisticated machine-learning algorithms to distill from its dataset a collection of trillions of searchable relationships across biology and chemistry unconstrained by human bias. By commanding massive experimental scale—up to millions of wet lab experiments weekly—and massive computational scale—owning and operating one of the most powerful supercomputers in the world—Recursion is uniting technology, biology, chemistry and patient-centric data to advance the future of medicine.
Recursion is headquartered in Salt Lake City, where it is a founding member of BioHive, the Utah life sciences industry collective. Recursion also has other primary offices in Toronto, Montreal, the San Francisco Bay Area, New York, the Oxford area, and London.
Recursion Investor Relations
[email protected]
Recursion Media
[email protected]
Forward Looking Statements
Statements contained herein which are not historical facts may be considered forward-looking statements under federal securities laws and may be identified by words such as "anticipates," "believes," "estimates," "expects," "intends," "plans," "potential," "predicts," "projects," "seeks," "should," "will," or words of similar meaning and include, but are not limited to, statements regarding the leadership position of the combined company and its impact on the industry; the ability for the combined business to accelerate the discovery of better solutions for patients; the timing of IND submissions and IND enabling studies; the potential to receive upfront, milestone, and royalty payments and work on over 60 therapeutic programs; the strengthening of the Recursion OS through the combined company; the continued learning of Recursion's platform and the creation of virtual cells to enable execution of clinical trials at scale; Recursion's achievement of efficiencies; the continuous expansion of the Recursion OS datasets; and advancing the future of medicine; the outlook for Recursion's future business and financial performance; and others. Such forward-looking statements are based on the current beliefs of Recursion's management as well as assumptions made by and information currently available to them, which are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Actual outcomes and results may vary materially from these forward-looking statements based on a variety of risks and uncertainties including: the ability of the combined company to retain key personnel; the ability to realize the benefits of the combination, including cost synergies; the ability to successfully integrate Exscientia's business with Recursion's business, at all or in a timely manner; the amount of the costs, fees, expenses and charges related to the combination; the effect of economic, market or business conditions, including competition, regulatory approvals and commercializing drug candidates, or changes in such conditions, have on the combined company's operations, revenue, cash flow, operating expenses, employee hiring and retention, relationships with business partners, the development or launch of technology enabled drug discovery, and commercializing drug candidates; the risks of conducting business internationally; the impact of changes in interest rates by the Federal Reserve and other central banks; the impact of potential inflation, volatility in foreign currency exchange rates and supply chain disruptions; the ability to maintain technology-enabled drug discovery in the biopharma industry; and risks relating to the market value of Recursion's Class A common stock.
Other important factors and information are contained in Recursion's most recent Annual Report on Form 10-K, including the risks summarized in the section entitled "Risk Factors," Recursion's subsequent Quarterly Reports on Form 10-Q, the joint definitive proxy statement filed by Recursion and Exscientia on October 10, 2024, as amended by the supplemental disclosures filed by Recursion on November 6, 2024, and each of Recursion's other filings with the U.S. Securities and Exchange Commission (the "SEC"), which can be accessed at https://ir.recursion.com, or www.sec.gov. All forward-looking statements are qualified by these cautionary statements and apply only as of the date they are made. Recursion undertakes no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/359d7cd4-0ccf-4210-938d-f585a9b073ee
https://www.globenewswire.com/NewsRoom/AttachmentNg/a94dc301-518d-48a8-b8d3-430cd726d651
https://www.globenewswire.com/NewsRoom/AttachmentNg/57cb9454-a7a7-4abf-a76f-82356a3682ac

