Skye Bioscience Demonstrates Over 30% Weight Loss with Nimacimab and Tirzepatide Combination in Preclinical Model
- Nimacimab shows comparable weight loss to monlunabant and tirzepatide alone, and an additive effect in combination with tirzepatide, in diet-induced obesity model
- New in vitro data demonstrates superior potency of nimacimab's differentiated and favorable mechanism of inhibition versus monlunabant
- Nimacimab Phase 2a CBeyond™ top-line randomized data expected late Q3/early Q4 2025
SAN DIEGO, April 15, 2025 (GLOBE NEWSWIRE) -- Skye Bioscience, Inc. (NASDAQ:SKYE) ("Skye"), a clinical-stage biotechnology company focused on unlocking new therapeutic pathways for obesity and other metabolic health disorders, today announced new preclinical data for its novel CB1 antibody, nimacimab. In a murine diet-induced obesity (DIO) model, after 25 days of treatment, results demonstrated:
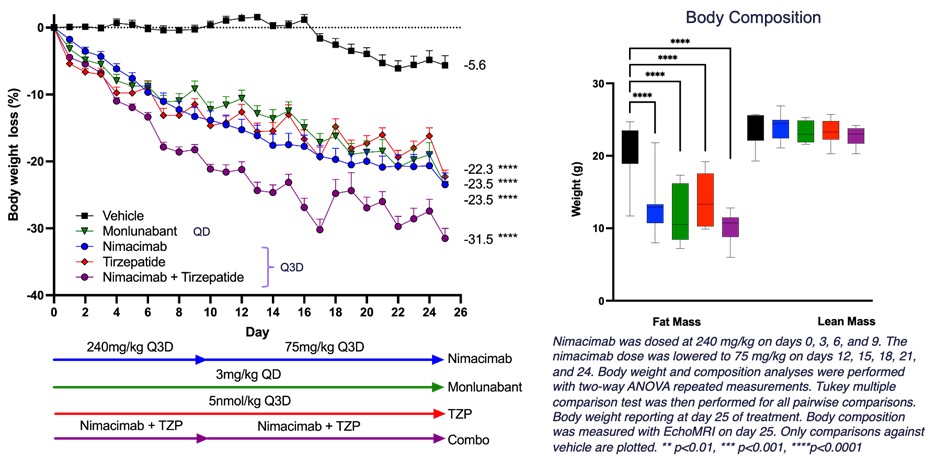
- Greater than 30% weight loss when nimacimab was combined with the dual GLP-1/GIP agonist, tirzepatide
- Nimacimab alone demonstrated 23.5% weight loss, comparable to monlunabant and tirzepatide alone.
"This new preclinical study highlights that a truly peripherally-restricted CB1 inhibitor—nimacimab—effectively drives weight loss in a DIO model. Nimacimab compared favorably to and provided significant additive weight loss when combined with GLP-1-targeted drugs like tirzepatide," said Punit Dhillon, CEO of Skye. "Using higher doses, this study builds on our previous preclinical DIO data in human CB1 knock-in mice that showed significant dose-dependent weight loss. Biomarker analyses demonstrated that nimacimab-driven weight loss was associated with beneficial changes in key hormones, glycemic control, and inflammatory markers.
"Skye believes nimacimab shows potential both as a monotherapy and in combination with a GLP-1 targeted drug to address unmet needs in obesity with the potential to change weight loss standards of care. Initial data from Skye's Phase 2a study in obesity is expected in late Q3/early Q4 2025."
Mr. Dhillon added, "The second key finding of this animal study is that Skye's highly-peripherally restricted nimacimab drives efficacy similar to a less-peripherally restricted CB1 inhibitor, monlunabant, in a DIO model. These in vivo data continue to support our belief that our differentiated antibody approach can potentially provide meaningful efficacy without the challenge current small molecule CB1 inhibitors face—brain exposure that can cause unwanted neuropsychiatric side effects."
Figure 1 - DIO model to interrogate combination of nimacimab and tirzepatide
New In Vitro Data Characterizes Differentiated Potency Characteristics
Skye also shared new in vitro potency data demonstrating that nimacimab's non-competitive allosteric binding to CB1 provides for a differentiated and potentially advantageous mechanism of inhibition versus small molecules like monlunabant, which must compete with CB1 agonists. In this study, potency of nimacimab and monlunabant were assessed against two concentrations of the CB1 agonist CP55940. The first condition evaluated potency of each drug with a lower concentration of CP55940 (50nM or EC80), while the second condition evaluated potency against an elevated concentration of CP55940 (2000nM or 40X EC80). These two conditions serve as a model of a physiological versus a pathological state where conditions such as obesity can promote an increase in the CB1 ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and thus competition for the CB1 receptor. These data demonstrated that while nimacimab's potency remained relatively stable, the activity of monlunabant when challenged with a higher concentration of a CB1 agonist was significantly impacted.
Dr. Chris Twitty, Chief Scientific Officer of Skye, said, "These data demonstrate for the first time how nimacimab's allosteric binding to the CB1 receptor is differentiated from the small molecules which bind to the receptor's active orthosteric site. We know that in a disease state such as obesity, the CB1 receptor as well as its natural ligands, AEA and 2-AG, are upregulated. In this diseased state, there may be significant competition for the active binding site. In our in vitro experiment we aimed to recreate this potential situation. The biological impact of these data suggests that when there is significant competition for CB1 binding, the activity of small molecules like monlunabant can be significantly impacted. Clinically this could result in impacting the relationship between pharmacodynamics and pharmacokinetics of the drug, ultimately requiring more of the small molecule to overcome the competition. Alternatively, nimacimab does not compete for the same site as the natural ligands, and our data show that as a result of this allosteric binding, the potency is minimally impacted regardless of the concentration of competing molecules."
"In the context of CB1 inhibition, we aim to realize the weight loss and metabolic benefits of this mechanism without the neuropsychiatric side effects seen with small molecule drugs. In our estimation, the potential of superior potency of nimacimab in this disease state may offer the widest possible therapeutic window among CB1 inhibitors."
Figure 2 - Comparison of potency between monlunabant* and nimacimab**
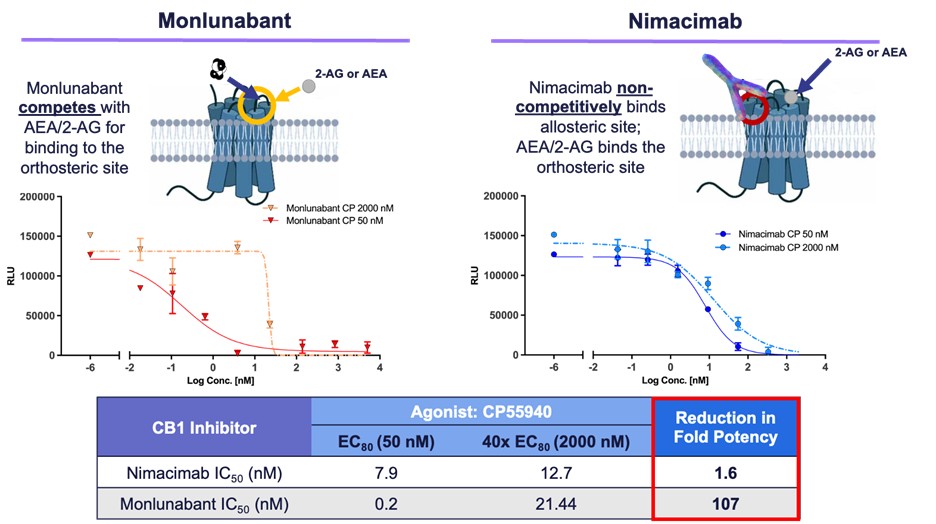
* Monlunabant's potency dropped significantly at high agonist levels due to direct competition for the receptor's orthosteric site.
** Nimacimab's potency was preserved due to its allosteric binding mechanism that avoids direct competition.
About Skye Bioscience
Skye is focused on unlocking new therapeutic pathways for metabolic health through the development of next-generation molecules that modulate G-protein coupled receptors. Skye's strategy leverages biologic targets with substantial human proof of mechanism for the development of first-in-class therapeutics with clinical and commercial differentiation. Skye is conducting a Phase 2 clinical trial (ClinicalTrials.gov: NCT06577090) in obesity for nimacimab, a negative allosteric modulating antibody that peripherally inhibits CB1. This study is also assessing the combination of nimacimab and a GLP-1R agonist (Wegovy®). For more information, please visit: www.skyebioscience.com. Connect with us on X and LinkedIn.
CONTACTS
Investor Relations
[email protected]
(858) 410-0266
LifeSci Advisors, Mike Moyer
[email protected]
(617) 308-4306
Media Inquiries
LifeSci Communications, Michael Fitzhugh
[email protected]
(628) 234-3889
FORWARD LOOKING STATEMENTS
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. In some cases, forward-looking statements can be identified by terminology including "anticipated," "plans," "goal," "focus," "aims," "intends," "believes," "can," "could," "challenge," "predictable," "will," "would," "may" or the negative of these terms or other comparable terminology. These forward looking statements include, but are not limited to: (i) statements regarding the superior safety and tolerability profile of nimacimab relative to other small molecule CB1 inhibitors, (ii) statements relating to any expectations regarding the efficacy and therapeutic potential of nimacimab as a monotherapy or in combination with a GLP-1 targeted drug, including expectations based on preclinical DIO models, (iii) statements regarding nimacimab's potential to change weight loss standards of care, (iv) statements regarding superior potency of nimacimab to other small molecule CB1 inhibitors based on nimacimab's mechanism of action and (v) statements regarding the timing of receipt of final data from Skye's Phase 2 obesity study of nimacimab. Such statements and other statements in this press release that are not descriptions of historical facts are forward-looking statements that are based on management's current expectations and assumptions and are subject to risks and uncertainties. If such risks or uncertainties materialize or such assumptions prove incorrect, our business, operating results, financial condition, and stock price could be materially negatively affected. We operate in a rapidly changing environment, and new risks emerge from time to time. As a result, it is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements the Company may make. Risks and uncertainties that may cause actual results to differ materially include, among others, our capital resources, uncertainty regarding the results of future testing and development efforts and other risks that are described in the Company's periodic filings with the Securities and Exchange Commission, including in the "Risk Factors" section of Skye's most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Except as expressly required by law, Skye disclaims any intent or obligation to update these forward-looking statements.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/97a10bc7-3e79-4fbc-9275-d0929f35c403
https://www.globenewswire.com/NewsRoom/AttachmentNg/acfb12e0-c08e-4700-b340-bc19d8a8a712


