Stelo by Dexcom, the First Over-the-Counter Glucose Biosensor in the U.S., Is Now Available
- Available for purchase today at Stelo.com for people in the U.S. 18 and older not using insulin
- Provides 24/7 powerful and personalized glucose insights directly to a smartphone,* revealing how food, exercise and sleep can affect glucose
- Tracking glucose just got easier, with no prescription and no fingersticks, ever
- Specifically designed to help people with Type 2 diabetes not using insulin and those with prediabetes reach their A1c goals and potentially slow the progression of diabetes.†,1-3
DexCom, Inc. (NASDAQ:DXCM), the global leader in real-time continuous glucose monitoring for people with diabetes, announced today that Stelo, the first over-the-counter glucose biosensor in the U.S., is now available for purchase without a prescription4 at Stelo.com.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240826019839/en/
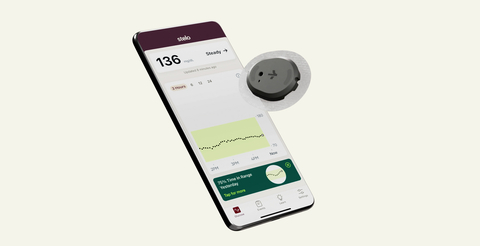
Stelo is designed to help people with Type 2 diabetes not using insulin and those with prediabetes. (Photo: Dexcom)
Stelo is a small biosensor worn on the back of the upper arm that leverages Dexcom's most accurate glucose sensing technology.5 It is specifically designed to provide the 125 million Americans6 with Type 2 diabetes not using insulin and those with prediabetes with powerful, personalized glucose insights sent directly to a smartphone,* revealing how food, exercise and sleep can affect glucose – all without painful fingersticks.
"Dexcom has been at the forefront of glucose biosensing for 25 years. With the launch of Stelo, we're defining a brand-new category and once again setting the gold standard for people to easily take control of their health," said Jake Leach, executive vice president and chief operating officer at Dexcom. "Now, millions more have access to 24/7, easy-to-understand glucose insights that can inform their daily lifestyle choices and support behavior modification."
The benefits of glucose biosensing have been shown when used alone, or alongside other diabetes and weight management medications.7 Studies show the use of Dexcom glucose biosensing by people with Type 2 diabetes is associated with clinically meaningful improvement in time in range, A1c and quality of life.8-11
"Dexcom glucose biosensors are an essential and proven tool for diabetes management – driving strong clinical outcomes regardless of medication use9 and even potentially slowing the progression of diabetes,†,1-3" said Thomas Grace, MD, head of clinical advocacy and outcomes at Dexcom. "In a world where GLP-1 use is becoming increasingly more common, glucose biosensors like Stelo can help make those medications more effective.12"
Key features of Stelo
- No fingersticks, ever: The only over-the-counter glucose biosensor designed for people with diabetes that doesn't require any fingersticks.
- Up to 15-day wear time‡ and waterproof§: The longest biosensor wear time on the market‡ with the highest waterproof rating.
- A personalized, easy-to-use app4: Provides daily, weekly and session summary insights that can help form healthier habits.
- Spike and pattern detection4: The only over-the-counter glucose biosensor for people with diabetes featuring spike detection, designed to identify meaningful glucose variability as it happens so users can make informed changes.
- Proven results with Dexcom glucose sensors†: Using Dexcom glucose sensors like Stelo helps lower A1c†,1,10, 13-19 for people with Type 2 diabetes and may help slow the progression of diabetes.†,1-3 Only Dexcom glucose sensors have been associated with significant improvements in glucose and cardiovascular risk reduction measures as early as three months.||,13
Effortless ordering, delivered directly to your door
Stelo improves access to critical health technology for people with Type 2 diabetes not using insulin and those with prediabetes who might not have insurance coverage for prescription glucose biosensors. Stelo is currently available for purchase in the U.S. at Stelo.com and is FSA and HSA eligible.
- Pay-as-you-go: Pay $99 for a single pack of two sensors (total wear time up to 30 days‡).
- Monthly subscription: Subscribe and save 10%. Pay $89 per month for an ongoing subscription, with two sensors (total wear time up to 30 days‡) delivered every 30 days.
Stelo is now part of Dexcom's overall portfolio of glucose biosensors, with a user base of more than 2.5 million people globally. The Dexcom portfolio in the U.S. consists of the Dexcom G6 and Dexcom G7 Continuous Glucose Monitoring Systems, and now Stelo, collectively designed to address the needs of people with all types of diabetes and prediabetes.
Each product in the portfolio is built for the people who use them, making it easier than ever for healthcare providers to get patients started with the glucose biosensor best for them. Dexcom G6 and Dexcom G7 are designed for people with diabetes using insulin or who are at risk of hypoglycemia and who have insurance coverage for glucose biosensors. Both systems require a prescription and are reimbursed by 97% of commercial insurers in the U.S., Medicare nationally and Medicaid in most states.5 Stelo is designed for adults with Type 2 diabetes not using insulin or prediabetes who seek behavior change and optimized health and who do not have insurance coverage for glucose biosensors.
Visit Stelo.com today to get started with Stelo, or do a benefits check to see if you are eligible for Dexcom G7 at Dexcom.com/Start.
About DexCom, Inc.
DexCom, Inc. empowers people to take control of health through innovative continuous glucose monitoring (CGM) systems. Headquartered in San Diego, Calif., and with operations across Europe and select parts of Asia/Oceania, Dexcom has emerged as a leader of diabetes care technology. By listening to the needs of users, caregivers and providers, Dexcom works to simplify and improve diabetes management around the world. For more information on Dexcom, visit https://www.dexcom.com/about.
*Smart device sold separately. For Stelo app compatibility information, visit stelo.com/compatibility. †Results obtained from previous Dexcom device(s) in people with diabetes who may be on insulin. ‡A study was conducted to assess the sensor life where 77.9% of sensors lasted the full 15 days. In other words, when using the product per the package labeling, approximately 20% of sensors may not last for the full 15 days, 10% of these sensors may last less than 12 days. §The Stelo biosensor is waterproof and may be submerged under eight feet of water for up to 24 hours without failure when properly installed. If you are in or near water, your smartphone may need to be closer than 20 feet to get readings. If you are in water, you may not get readings until you get out. ||Cardiovascular risk refers to 10-year ASCVD risk. Visit https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/ to access the American College of Cardiology ASCVD Risk Estimator Plus.
1 UKPDS Group. Lancet.1998;352(9131):837-53. 2 Battelino T, et al. Diabetes Care. 2019;42(8):1593-603. 3 Vigersky RA, et al. Diabetes Technol Ther. 2019;21(2):81-85. 4 Stelo User Guide. 5 Dexcom, data on file, 2024. 6 Centers for Disease Control and Prevention. National Diabetes Statistics Report. https://www.cdc.gov/diabetes/php/data-research/index.html. Accessed on August 12, 2024. 7 Grace T, Salyer J. Diabetes Technol Ther. 2022 Jan;24(1):26-31. 8 Cox DJ, Banton T, Moncrief M, et al. J Endocr Soc 2020;4(11):bvaa118. 9 Hannah K, Nemlekar P, Norman G. Improved glycemic control after real-time continuous glucose monitor (rtCGM) initiation in patients with type 2 diabetes (PWT2D) stratified by insulin therapy and race/ethnicity. Presented at the 17th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2024), March 6-9, 2024, Florence, Italy and online. 10 Jepson LH, Welsh J, Green CR, et al. Diabetes 2023;72(Supplement_1):941-P. 11 Layne J, Jepson, Thomas R, et al. Non-insulin treated adults with type 2 diabetes benefit from CGM use: real-world data. Presented at the 17th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2024), March 6-9, 2024, Florence, Italy and online. 12 Nemlekar et al. Diabetes. 2024; 73 (Supplement_1): 1914–LB. 13 Reed J, et al. Diabetes Obes Metab. 2024;26(7):2881-9. 14 Polonsky W, et al. ATTD. 2024;O013(535). 15 Beck RW, et al. JAMA. 2017;317(4):371-378. 16 Beck RW, et al. Ann Intern Med. 2017;167(6):365-374. 17 Martens T, et al. JAMA. 2021;325(22):2262-2272. 18 Laffel LM, et al. JAMA. 2020;323(23):2388-2396. 19 Welsh JB, et al. J Diabetes Sci Technol. 2024;18(1):143-7.
STELO IMPORTANT INFORMATION: Consult your healthcare provider before making any medication adjustments based on your sensor readings and do not take any other medical action based on your sensor readings without consulting your healthcare provider. Do not use if you have problematic hypoglycemia. Failure to use Stelo and its components according to the instructions for use provided and to properly consider all indications, contraindications, warnings, and cautions in those instructions for use may result in you missing a severe hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose) occurrence. If your sensor readings are not consistent with your symptoms, a blood glucose meter may be an option as needed and consult your healthcare provider. Seek medical advice and attention when appropriate, including before making any medication adjustments and/or for any medical emergency.
INDICATIONS FOR USE: The Stelo Glucose Biosensor System is an over-the-counter (OTC) integrated Continuous Glucose Monitor (iCGM) intended to continuously measure, record, analyze, and display glucose values in people 18 years and older not on insulin. The Stelo Glucose Biosensor System helps to detect normal (euglycemic) and low or high (dysglycemic) glucose levels. The Stelo Glucose Biosensor System may also help the user better understand how lifestyle and behavior modification, including diet and exercise, impact glucose excursion. The user is not intended to take medical action based on the device output without consultation with a qualified healthcare professional.
Dexcom, Dexcom Clarity, Dexcom Follow, Dexcom One, Dexcom Share, Stelo, and any related logos and design marks are either registered trademarks or trademarks of Dexcom, Inc. in the United States and/or other countries. ©2024 Dexcom, Inc. All rights reserved.
Category: IR
View source version on businesswire.com: https://www.businesswire.com/news/home/20240826019839/en/
