Aclaris Therapeutics' Novel ITK/JAK3 Inhibitor ATI-2138 Demonstrates Rapid and Sustained Hair Regrowth in Validated Murine Model of Alopecia Areata (AA)
- ATI-2138 Outperforms Ritlecitinib in Reversal Model of Alopecia Universalis, the Most Severe AA Phenotype -
WAYNE, Pa., Jan. 27, 2026 (GLOBE NEWSWIRE) -- Aclaris Therapeutics, Inc. (NASDAQ:ACRS), a clinical-stage biopharmaceutical company focused on developing novel product candidates for immuno-inflammatory diseases, today announced recent positive preclinical results from its potent and selective ITK/JAK3 inhibitor ATI-2138 in a murine model of severe alopecia areata (AA). In this model of hair loss, conducted by Dr. Angela Christiano at Columbia University, ATI-2138 demonstrated rapid, near complete, and sustained hair regrowth compared to control and ritlecitinib.
"ATI-2138 is a potent and selective inhibitor of ITK and JAK3 with dual pharmacology that has been demonstrated to downregulate Th1, Th2, Th17, TCR (ITK) and fibrosis pathway markers, and reduce itch; this makes it an excellent mechanistic fit in a variety of I&I diseases, including across the spectrum of inflammatory alopecias," said Dr. Roland Kolbeck, Chief Scientific Officer of Aclaris. "The mice used in this model of severe alopecia areata were significantly older and more difficult to treat than those typically studied; despite that, ATI-2138 induced an accelerated response and near complete regrowth after only four weeks that was sustained through the end of study, and outperformed ritlecitinib."
Continued Dr. Kolbeck, "The murine model of alopecia areata has been well-validated and is highly predictive of clinical responses in human trials of JAK inhibitors, including ritlecitinib. The rapid onset of hair regrowth in mice treated with ATI-2138 is compelling, since this drug is differentiated from other JAK inhibitors due to its dual targeting of ITK as well as JAK3."
ATI-2138 is an investigational oral compound that interrupts T cell receptor (TCR) signaling by inhibiting ITK and JAK3 signaling of IL-2 receptor common gamma chain (IL-2Rγc) cytokines in lymphocytes (including IL-2, IL-4, and IL-15), thus regulating T cell expansion, differentiation and activation upstream (ITK) and downstream (JAK3). It is highly potent for both ITK and JAK3, and more than 1,000-fold selective against other JAK isoforms. This unique pharmacology may provide a highly potent and complete anti-inflammatory response. ATI-2138 has the potential to treat a variety of inflammatory, atopic, and autoimmune diseases, including alopecias (hair loss).
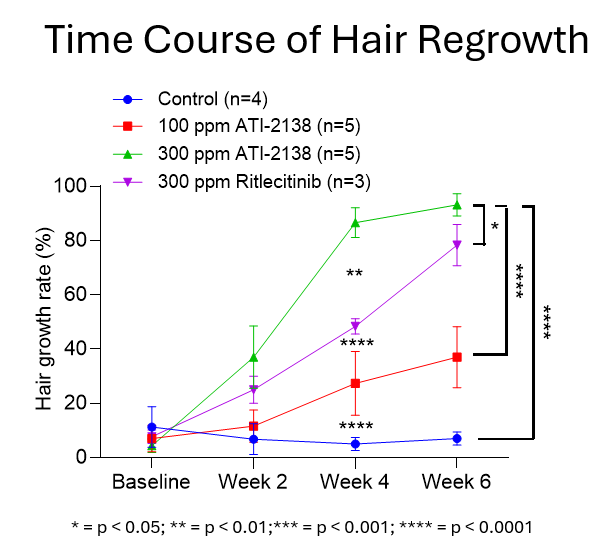
ATI-2138 and ritlecitinib were assessed using a reversal model of murine alopecia universalis, the most severe AA phenotype. Mice were randomized to control or drug formulated in chow (100 or 300 ppm ATI-2138; 300 ppm ritlecitinib) after hair loss was established. Hair regrowth was assessed at baseline and weeks 2, 4, and 6.
- Rapid onset of hair regrowth was observed at week 2 in mice receiving 300 ppm ATI-2138 (mean percent hair regrowth of 37%) and reached near peak effect at week 4 (mean percent hair regrowth of 87%) compared to 300 ppm ritlecitinib (25% and 48%, respectively). Mice receiving control chow showed no improvement in hair regrowth.
- At week 6, the mean percent hair regrowth was 93% for ATI-2138 (300 ppm) compared to 78% for ritlecitinib (300 ppm). Mice receiving control chow showed no improvement in hair regrowth.
Given the uniquely potent and selective mechanism of ATI-2138, Aclaris is assessing additional indications for ATI-2138, including alopecias, and expects to initiate a Phase 2b trial in the first half of 2026.
About ATI-2138
ATI-2138 is a highly potent and selective novel investigational pharmacologic agent that acts as a dual inhibitor of interleukin-2-inducible T cell kinase (ITK) and Janus kinase 3 (JAK3). ITK regulates T cell receptor signal transduction and inhibition of this kinase can affect T cell differentiation and activation. JAK3 is a key signal transduction kinase that forms a heterodimer with JAK1, modulates JAK1 phosphorylation of signal transducer and activator of transcription 5 (STAT5), and regulates cytokines that signal through the IL-2Rγc to affect lymphocyte proliferation and activation. The efficacy results exhibited in preclinical animal models of inflammation and autoimmune diseases, coupled with the favorable safety, pharmacokinetics, and pharmacodynamics profile in healthy human SAD and MAD studies and a Phase 2a trial in atopic dermatitis, support the potential for ATI-2138 to affect several human inflammatory diseases and further investigation of this molecule in patients with atopic and autoimmune diseases that are dependent on T cell function and/or IL-2Rγc signaling.
About Aclaris Therapeutics, Inc.
Aclaris Therapeutics, Inc. is a clinical-stage biopharmaceutical company developing a pipeline of novel product candidates to address the needs of patients with immuno-inflammatory diseases who lack satisfactory treatment options. The company has a multi-stage portfolio of product candidates powered by a robust R&D engine. For additional information, please visit www.aclaristx.com and follow Aclaris on LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as "anticipate," "believe," "expect," "intend," "may," "plan," "potential," "will," and similar expressions, and are based on Aclaris' current beliefs and expectations. These forward-looking statements include expectations regarding its development plans for ATI-2138, including the timing of initiating a Phase 2b trial and the potential indication for such trial, and the therapeutic potential for ATI-2138, including in inflammatory, atopic, and autoimmune diseases including alopecias. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, Aclaris' reliance on third parties over which it may not always have full control, Aclaris' ability to enter into strategic partnerships on commercially reasonable terms, the uncertainty regarding the macroeconomic environment and other risks and uncertainties that are described in the Risk Factors section of Aclaris' Annual Report on Form 10-K for the year ended December 31, 2024, and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the "SEC Filings" page of the "Investors" section of Aclaris' website at www.aclaristx.com. Any forward-looking statements speak only as of the date of this press release and are based on information available to Aclaris as of the date of this release, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise.
Aclaris Therapeutics Contact:
Will Roberts
Senior Vice President
Corporate Communications and Investor Relations
(484) 329-2125
[email protected]
A graphic accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/84a52e9f-e571-47d3-ada2-8c947c90a3b7


