Pliant Therapeutics Announces Positive Safety and Exploratory Efficacy Data from the INTEGRIS-PSC Phase 2a Trial of Bexotegrast 320 mg in Patients with Primary Sclerosing Cholangitis and Suspected Liver Fibrosis
Bexotegrast (PLN-74809) at 320 mg was well tolerated over 12 weeks of treatment with no drug-related severe or serious adverse events; No safety concerns identified across all dose cohorts
Bexotegrast at 320 mg reduced liver fibrosis markers ELF and PRO-C3 and showed improvements in hepatocyte function and bile flow by contrast MRI imaging relative to placebo at Week 12
The 320 mg data continue to demonstrate antifibrotic effects of bexotegrast, consistent with previous findings
Company to host webcast and conference call tomorrow, Monday, February 5 at 8:00 a.m. ET
SOUTH SAN FRANCISCO, Calif., Feb. 04, 2024 (GLOBE NEWSWIRE) -- Pliant Therapeutics, Inc. (NASDAQ:PLRX) today announced 12-week interim data from the 320 mg dose group of INTEGRIS-PSC, a multinational, randomized, double-blind, placebo-controlled Phase 2a clinical trial of bexotegrast in patients with primary sclerosing cholangitis (PSC) and suspected moderate to severe liver fibrosis. The 320 mg group met its primary and secondary endpoints demonstrating that bexotegrast was well tolerated over a 12-week treatment period and its plasma concentrations increased with dose. There was no dose relationship for adverse events. Pruritus and cholangitis occurred less frequently on bexotegrast than on placebo.
The trial's exploratory efficacy endpoints assessed changes in the liver fibrosis markers, Enhanced Liver Fibrosis (ELF) score and PRO-C3 levels, as well as liver biochemistry and magnetic resonance imaging (MRI) of the liver. Consistent with the results from the lower doses tested, bexotegrast-treated patients at the 320 mg dose showed a reduction in both ELF score and PRO-C3 levels relative to placebo at Week 12. Bexotegrast-treated patients also showed stabilization of alkaline phosphatase (ALP) levels, relative to an increase on placebo at Week 12. In addition, MRI imaging continued to show evidence of improved hepatocyte function and bile flow with bexotegrast at the 320 mg dose relative to placebo.
INTEGRIS-PSC is a multinational, randomized, dose-ranging, double-blind, placebo-controlled Phase 2a trial evaluating bexotegrast at once-daily oral doses of 40 mg, 80 mg, 160 mg, 320 mg or placebo for 12 weeks in 121 patients with PSC. The 320 mg group enrolled 27 patients in the active arm and added 9 new patients to the pooled placebo arm. We believe INTEGRIS-PSC to be the first randomized clinical trial to use an enrichment strategy to enroll patients with suspected moderate to severe liver fibrosis based on liver stiffness measure, ELF score or historical liver biopsy. Baseline characteristics of the trial population reflected this enrichment. The 320 mg dose group will continue until all patients have been treated for at least 24 weeks, with final data expected in mid-2024.
"Results from INTEGRIS-PSC continue to build on the favorable safety and tolerability data for bexotegrast which is critically important in the setting of vulnerable patient populations and the need for chronic therapies," said Éric Lefebvre, M.D., Chief Medical Officer of Pliant. "As the therapeutic profile of bexotegrast comes into focus with these data, it's encouraging to see bexotegrast's treatment effects manifested across multiple endpoints, suggesting its potential to impact PSC where therapies are urgently needed. I look forward to additional data from this trial in mid-2024 and upcoming discussions with regulatory authorities surrounding potential next steps."
Bexotegrast 320 mg was Well Tolerated with No Drug-Related Severe or Serious Adverse Events
The primary endpoint of the INTEGRIS-PSC trial is the evaluation of the safety and tolerability of bexotegrast. The secondary endpoint is an assessment of its pharmacokinetics.
Bexotegrast at the 320 mg dose was well tolerated with no dose relationship observed for adverse events. Of the 27 patients treated with bexotegrast at the 320 mg dose, 26 (96%) completed 12 weeks of treatment with no drug-related severe or serious adverse events (SAE). Most treatment-emergent adverse events (TEAEs) were mild or moderate in severity and consistent with PSC disease symptoms. In addition, adverse events of pruritus and cholangitis occurred less frequently on all doses of bexotegrast relative to placebo. Patients in the trial who had concomitant inflammatory bowel disease (IBD) saw no change in their IBD symptoms as measured by partial Mayo Score while on treatment.
Bexotegrast total and unbound plasma concentrations increased with dose.
Bexotegrast 320 mg Demonstrated Antifibrotic Activity in a PSC Population with Suspected Moderate to Severe Liver Fibrosis at Week 12
The exploratory endpoints of the INTEGRIS-PSC trial include changes in liver fibrosis markers, ELF and PRO-C3, liver biochemistry and MRI imaging.
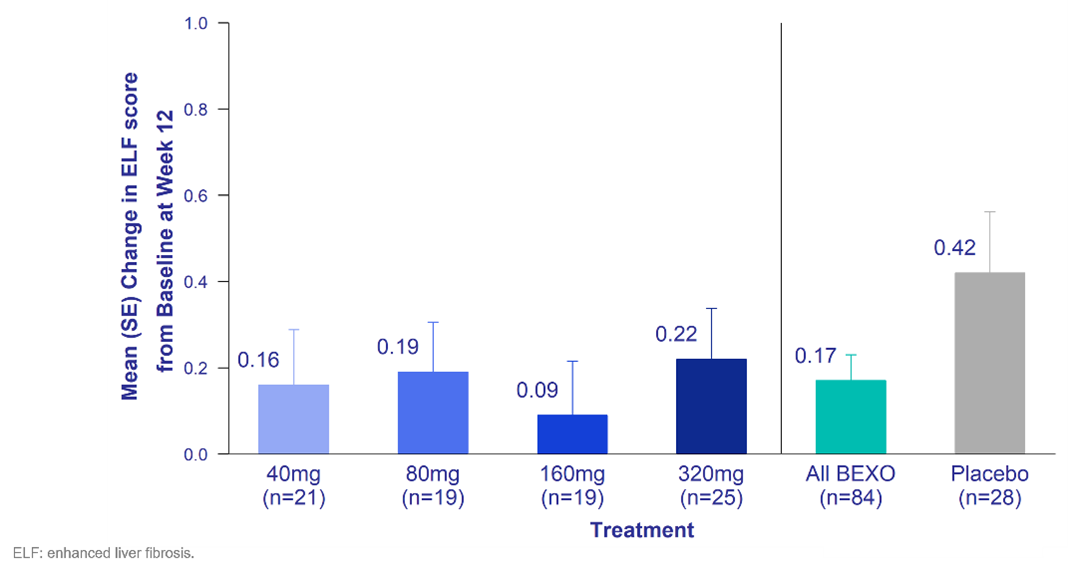
Figure 1. ELF Score – Change from Baseline at Week 12
Consistent with the lower doses tested, bexotegrast at 320 mg reduced ELF score relative to placebo at Week 12. The ELF score is a well-established prognostic marker of liver disease severity and liver-related events in patients with advanced fibrosis.1 ELF is strongly associated with transplant‐free survival in PSC and may be useful as a surrogate marker in clinical trials.2
Consistent with the lower doses tested, bexotegrast at 320 mg reduced PRO-C3 levels relative to placebo. PRO-C3 is a biomarker of active fibrogenesis with higher levels associated with greater disease activity.3
MRI relative enhancement using gadoxetate contrast is a measure of hepatocyte function, with increased enhancement suggesting improved hepatocyte function.4,5 Consistent with the lower doses tested, bexotegrast at the 320 mg dose showed an increase in relative enhancement on contrast MRI compared to a decrease observed in the placebo group at Week 12. In addition, consistent with the lower doses tested, bexotegrast at the 320 mg dose reduced time to arrival to the common bile duct compared to placebo, suggesting improved bile flow.6
Patients with PSC often experience pruritus, or itch, as part of their disease.7 Bexotegrast at the 320 mg dose demonstrated statistically significant reductions in the Itch Numerical Rating Scale relative to placebo at Week 12.
"Consistent with prior observations from the INTEGRIS-PSC trial, bexotegrast continues to demonstrate a very favorable safety profile while also maintaining efficacy signals," said Kris V. Kowdley MD, AGAF, FAASLD, FACP, FACG, Director, Liver Institute Northwest and Professor of Medicine, Elson S. Floyd College of Medicine at Washington State University. "These promising results present a strong rationale for the further study of bexotegrast in patients with PSC as part of a larger late-stage trial."
Bexotegrast in PSC Clinical Development Next Steps
The Company is planning to share these data from the INTEGRIS-PSC trial with regulatory authorities to discuss the potential path to registration.
We would like to thank our INTEGRIS-PSC investigators and their study teams for their dedication in support of the successful execution of this trial. Special thanks to the INTEGRIS-PSC clinical trial participants, their families and support networks for helping us advance this promising program.
Background on Primary Sclerosing Cholangitis
PSC is a rare, progressive liver disease of unknown origin, which frequently occurs in the setting of inflammatory bowel disease. PSC affects more than 30,000 patients in the United States and over 100,000 patients worldwide. The disease can occur in all ages, genders, and races. PSC is characterized by inflammation and fibrosis, with progressive liver and biliary damage leading to cirrhosis and liver failure. Currently there are no FDA or EMA-approved therapies for patients with PSC. Therefore, there is a high unmet need for new therapeutic options to address the symptoms and modify the disease progression of this grievous illness.
INTEGRIS-PSC Multinational Phase 2a Trial of Bexotegrast (NCT04480840)
INTEGRIS-PSC is a Phase 2a, multinational randomized, dose-ranging, double-blind, placebo-controlled trial evaluating the safety, tolerability, and pharmacokinetics of bexotegrast administered over 12 weeks in patients with IPF. Patients were enrolled in doses of 40 mg, 80 mg, 160 mg or 320 mg, with a 3:1 randomization ratio (active:placebo) and stratification based on use of ursodeoxycholic acid (UDCA). The primary endpoint is the evaluation of bexotegrast safety and tolerability and the secondary endpoint is the assessment of pharmacokinetics across the range of doses. Exploratory endpoints will measure changes in liver fibrosis markers, ELF and PRO-C3, liver biochemistry and liver imaging.
Conference Call and Webcast Information
The Company will host a conference call and webcast with a slide presentation tomorrow, Monday, February 5, 2024, at 8:00 a.m. ET | 5:00 a.m. PT to discuss this update. Members of Pliant's management team will be joined by Gideon Hirshfield, FRCP, Ph.D., Lily and Terry Horner Chair in Autoimmune Liver Disease at the University of Toronto. Interested parties may access the live webcast on Pliant's website at Pliant Therapeutics INTEGRIS-PSC Webcast or may participate via telephone by registering in advance at the following link: Pliant Therapeutics INTEGRIS-PSC Conference Call. Upon registration, all telephone participants will receive the dial-in number along and a unique passcode to access the call. An archived replay of the webcast will be available on Pliant's website for 60 days following completion of the event.
About Pliant Therapeutics, Inc.
Pliant Therapeutics is a clinical-stage biopharmaceutical company and leader in the discovery and development of novel therapeutics for the treatment of fibrotic diseases. Pliant's lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual selective inhibitor of αvß6 and αvß1 integrins that is in development in the lead indications for the treatment of idiopathic pulmonary fibrosis, or IPF, and primary sclerosing cholangitis, or PSC. Bexotegrast has received Fast Track Designation and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) in IPF and PSC and Orphan Drug Designation from the European Medicines Agency in IPF and PSC. Pliant has initiated BEACON-IPF, a Phase 2b trial of bexotegrast in IPF. Pliant has also developed PLN-1474, a small molecule, selective inhibitor of αvß1 integrin for the treatment of nonalcoholic steatohepatitis, or NASH with liver fibrosis. Pliant has initiated a Phase 1 study for its third clinical program, PLN-101095, a small molecule, dual-selective inhibitor of αvß8 and αvß1 integrins, that is being developed for the treatment of solid tumors. In addition to clinical-stage programs, Pliant currently has a preclinical program targeting muscular dystrophies. For additional information, please visit: www.PliantRx.com. Follow us on social media X, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. These statements include those regarding the safety, tolerability, pharmacodynamics and therapeutic potential of bexotegrast; our plans for the future development of bexotegrast; bexotegrast's potential to become a treatment for IPF or PSC; the anticipated timing of data and progress from our clinical studies; including the timing of 24-week data from the 320 mg dose cohort of the INTEGRIS-PSC Phase 2a trial in mid-2024; and discussions and interactions with regulatory authorities, including regarding a potential path to registration. Because such statements deal with future events and are based on our current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Pliant Therapeutics could differ materially from those described in or implied by the statements in this press release. These forward-looking statements are subject to risks and uncertainties, including those related to the development and commercialization of our product candidates, including any delays in our ongoing or planned preclinical or clinical trials, the impact of current macroeconomic and marketplace conditions, our reliance on third parties for critical aspects of our development operations, the risks inherent in the drug development process, the risks regarding the accuracy of our estimates of expenses and timing of development, our capital requirements and the need for additional financing, including the availability of additional term loans under our loan facility, and our ability to obtain and maintain intellectual property protection for our product candidates. These and additional risks are discussed in the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Quarterly Report on Form 10-Q for the period ended September 30, 2023 which is available on the SEC's website at www.sec.gov. Unless otherwise noted, Pliant is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
Investor and Media Contact:
Christopher Keenan
Vice President, Investor Relations and Corporate Communications
Pliant Therapeutics, Inc.
[email protected]
1 Vesterhus M, et al. Hepatology. 2019 69(2):684-698.
2 Bowlus CL, et al. Hepatology. 2023 Feb 1;77(2):659-702.
3 Nielsen MJ, et al. Aliment Pharmacol Ther. 2018 Jul;48(2):179-189.
4 Schulze J, et al. Clin Gastroenterol Hepatol. 2019 Jan;17(1):192-199.
5 Nilsson H, et al. J Magn Reson Imaging. 2014 Apr;39(4):879-86.
6 Elkilany A, et al. Abdom Radiol (NY). 2021 Mar;46(3):979-991.
7 Karlsen TH, et al. J Hepatol. 2017 Dec;67(6):1298-1323.


