Trinity Biotech Unveils CGM+: An AI-Native Platform Targeting the $260 Billion AI Wearable Market
WILSONVILLE, Ore. and DUBLIN, July 24, 2025 (GLOBE NEWSWIRE) -- Trinity Biotech plc (NASDAQ:TRIB), a commercial-stage biotechnology company focused on human diagnostics and diabetes management solutions, including wearable biosensors, today unveiled its new flagship product, CGM+, a next-generation wearable biosensor platform designed for the $260 billion AI wearables market. Now in the later stages of device development, CGM+ is Trinity's new AI-native continuous glucose monitoring (CGM) system, combining multi-sensor data and real-time analytics to meet the evolving demands of AI-powered healthcare and wellness.

CGM+ combines sustainability with user-friendly design, featuring a compact wearable and reusable sensor applicator.
Unlike traditional CGMs focused solely on glucose monitoring, CGM+ integrates an ultra-thin minimally invasive electrochemical glucose sensor with continuous monitoring of heart activity, body temperature and physical activity—all within a single, sleek and user-friendly modular wearable device. This multimodal data stream is being optimized for real-time AI analysis, to enable a deeper, more contextual understanding of metabolic and physiological health. Trinity Biotech has identified these expanded data points as critical to gain insights into essential metabolic factors, including sleep, stress, and physical activity. Trinity Biotech's vision is to combine medical-grade monitoring and consumer wellness insights, through the CGM+ platform, positioning Trinity Biotech at the forefront of a new category of intelligent wearables.
Trinity Biotech's proprietary needle-free glucose sensor technology facilitates the collection of expanded data points from a single modular wearable device. The Company's latest innovative design utilises this technology to provide a solution that not only reduces the number of disposable components and waste, but also significantly lowers the cost of care compared to current leading market products.
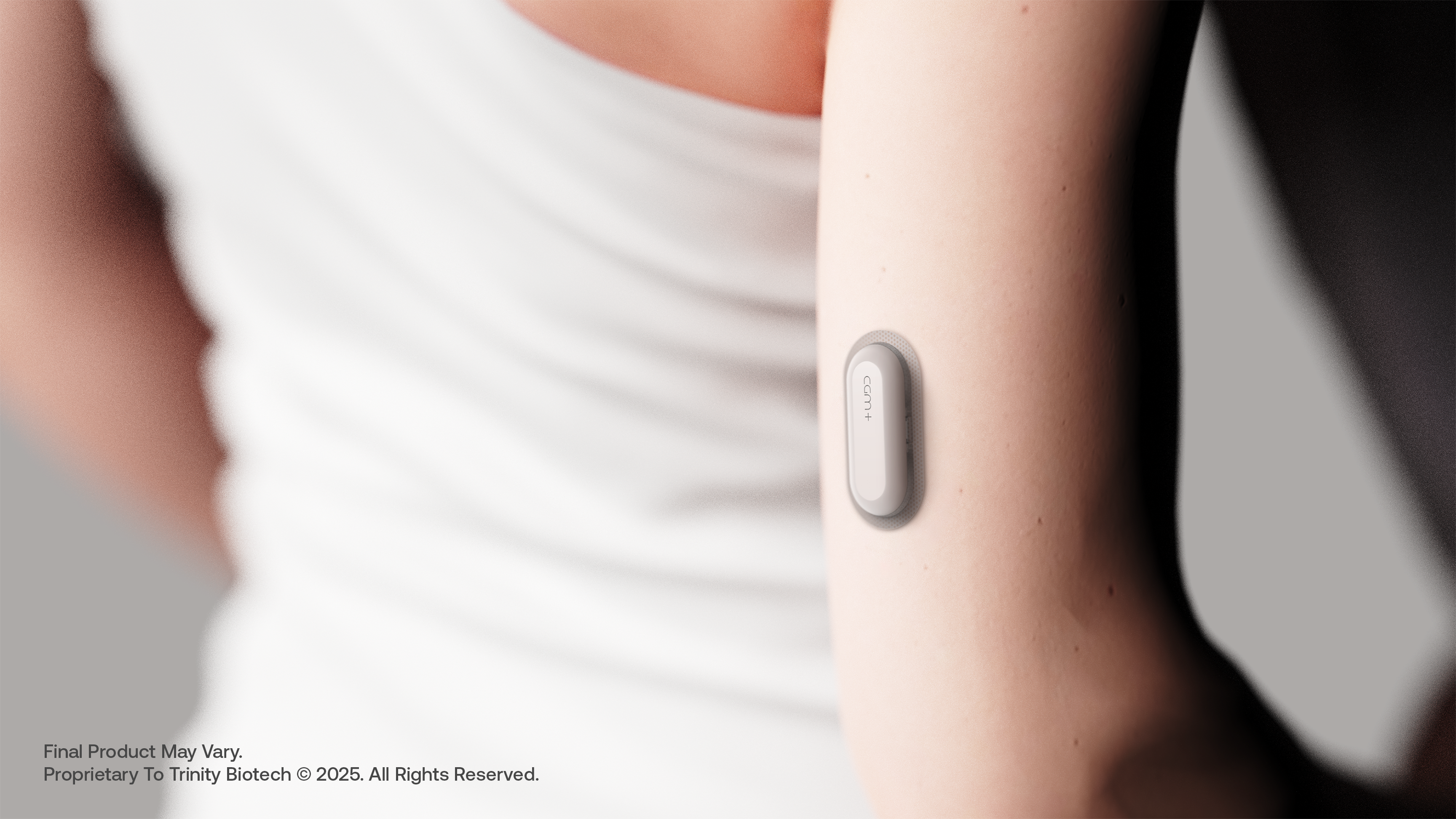
Modular wearable engineered for real-time, AI-native insights—CGM+ designed to integrate glucose, heart activity, temperature, and motion tracking in one seamless, sustainable wearable.
"CGM+ is not just a device—it's a proprietary data engine we are building for the AI health ecosystem," said John Gillard, CEO of Trinity Biotech. "We believe this technology can power the next wave of personalized, predictive, and preventative care—while opening up entirely new commercial pathways for Trinity Biotech, from device sales to AI-driven data services. It will also position us to compete well beyond traditional diagnostics, at the intersection of chronic disease management, digital health and consumer wellness."

Trinity Biotech's CGM+ wearable biosensor designed to seamlessly integrate glucose, cardiovascular, temperature, and activity monitoring in one sleek modular device.
Purpose-Built for Real-Time AI-Driven Healthcare
As healthcare shifts toward precision medicine and continuous, real-world data collection, CGM+ is positioned to become a critical enabler of AI-based diagnostics, behavioural coaching, and chronic disease management. Now in the later stages of device development, CGM+ has been engineered from the ground up to serve as a foundational platform for AI-native health applications:
- Comprehensive Multi-sensor Intelligence: Combines glucose, cardiovascular, thermoregulation and physical activity data for a holistic view of health and wellness.
- Clinical and Consumer Lifestyle Use Cases: Designed to support both regulated clinical workflows and consumer wellness applications, bridging the gap between medical-grade monitoring and everyday health optimization.
Market Opportunity
- Global CGM Market: Projected to grow from $13.28B in 2025 to $28.72B by 2030 (CAGR: 16.68%)1
- AI in Healthcare: Forecast to reach approximately $200B by 2030, growing at a 37.6% CAGR2
- Wearable AI: Expected to surpass $260B by 2032, growing at a 27.0% CAGR3
CGM+ is uniquely positioned at the intersection of these high-growth sectors, and is being designed to offer a differentiated solution for both clinical and consumer health markets.
Strategic Outlook and Additional Revenue Opportunities
Trinity Biotech anticipates commercial launch of CGM+ in mid-2026, opening multiple revenue streams, including device sales, AI analytics subscriptions and strategic partnerships with healthcare providers, insurers and digital health platforms.
"CGM+ is the cornerstone of our vision for AI-native health monitoring," added Gillard. "We're not just adding a product—we're building a scalable platform with recurring revenue potential that we believe can drive long-term growth across clinical and consumer markets."
Trinity Biotech recently completed a further pre-pivotal trial of its upgraded glucose sensor technology and expects to issue key findings from this trial shortly.
For further information, please see the Company's CGM+ microsite: https://cgm.trinitybiotech.com
1 https://www.mordorintelligence.com/industry-reports/continuous-glucose-monitoring-market
2 https://www.mordorintelligence.com/industry-reports/artificial-intelligence-in-healthcare-market
3 https://www.fortunebusinessinsights.com/wearable-ai-market-109561
Forward-Looking Statements
This release includes statements that constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 (the "Reform Act"), including but not limited to statements related to Trinity Biotech's cash position, financial resources and potential for future growth, market acceptance and penetration of new or planned product offerings, and future recurring revenues and results of operations. Trinity Biotech claims the protection of the safe harbor for forward-looking statements contained in the Reform Act. These forward-looking statements are often characterized by the terms "may," "believes," "projects," "expects," "anticipates," or words of similar import, and do not reflect historical facts. Specific forward-looking statements contained in this release may be affected by risks and uncertainties, including, but not limited to, our ability to capitalize on the Waveform transaction and of our recent acquisitions, our continued listing on the Nasdaq Stock Market, our ability to achieve profitable operations in the future, the impact of the spread of COVID-19 and its variants, potential excess inventory levels and inventory imbalances at the company's distributors, losses or system failures with respect to Trinity Biotech's facilities or manufacturing operations, the effect of exchange rate fluctuations on international operations, fluctuations in quarterly operating results, dependence on suppliers, the market acceptance of Trinity Biotech's products and services, the continuing development of its products, required government approvals, risks associated with manufacturing and distributing its products on a commercial scale free of defects, risks related to the introduction of new instruments manufactured by third parties, risks associated with competing in the human diagnostic market, risks related to the protection of Trinity Biotech's intellectual property or claims of infringement of intellectual property asserted by third parties and risks related to condition of the United States economy and other risks detailed under "Risk Factors" in Trinity Biotech's annual report on Form 20-F for the fiscal year ended December 31, 2024 and Trinity Biotech's other periodic reports filed from time to time with the United States Securities and Exchange Commission. Forward-looking statements speak only as of the date the statements were made. Trinity Biotech does not undertake and specifically disclaims any obligation to update any forward-looking statements.
About Trinity Biotech
Trinity Biotech is a commercial stage biotechnology company focused on diabetes management solutions and human diagnostics, including wearable biosensors. The Company develops, acquires, manufactures and markets diagnostic systems, including both reagents and instrumentation, for the point-of-care and clinical laboratory segments of the diagnostic market and has recently entered the wearable biosensor industry, with the acquisition of the biosensor assets of Waveform Technologies Inc. and intends to develop a range of biosensor devices and related services, starting with a continuous glucose monitoring product. Our products are used to detect infectious diseases and to quantify the level of Haemoglobin A1c and other chemistry parameters in serum, plasma and whole blood. Trinity Biotech sells direct in the United States and through a network of international distributors and strategic partners in over 75 countries worldwide. For further information, please see the Company's website: www.trinitybiotech.com.
| Contact: | Trinity Biotech plc | RedChip Companies Inc. |
| Gary Keating, Ph.D | Dave Gentry, CEO | |
| (353)-1-2769800 | (1)-407-644-4256 | |
| [email protected] | ||
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/03ec64b3-baf3-4d1c-8323-ddb34d9f333c
https://www.globenewswire.com/NewsRoom/AttachmentNg/728e5d44-7d29-4326-ad0e-73912c0b5b6a
https://www.globenewswire.com/NewsRoom/AttachmentNg/fdc94876-019a-4efc-9db0-552858dcdf77

