Zai Lab Highlights Strategic Priorities and Global Pipeline Progress at 44th Annual J.P. Morgan Healthcare Conference
- Zocilurtatug pelitecan (Zoci) positioned to become Zai Lab's first global oncology launch, with three registration-enabling studies across 2L+ SCLC, 1L SCLC and NEC by the end of 2026
- Rapidly advancing a differentiated global pipeline, including ZL-1503 (IL-13xIL-31), ZL-6201 (LRRC15 ADC), ZL-1222 (PD-1xIL-12) and ZL-1311 (MUC17xCD3)
- COBENFY approved in China, with commercial launch expected in 1H 2026
- Zai Lab to present at the J.P. Morgan Annual Healthcare Conference on Tuesday, January 13, 2026, at 3:00 p.m. PT / 6:00 p.m. ET
Zai Lab Limited (NASDAQ:ZLAB, HKEX: 9688)) today provided an update on the Company's 2026 strategic priorities and clinical development progress. These updates will be discussed as part of the Company's presentation at the 44th Annual J.P. Morgan Healthcare Conference in San Francisco on Tuesday, January 13, 2026, at 3:00 p.m. PT / 6:00 p.m. ET.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260113130363/en/
"Since our founding, we have intentionally built Zai Lab as a dual-engine company – combining a commercially profitable and scaling China business with a global innovation engine that is now reaching a pivotal inflection point," said Samantha Du, Founder, Chairperson, and CEO of Zai Lab. "With Zoci now in Phase 3 and multiple global programs advancing behind it, we are entering our next phase with a highly efficient, globally integrated R&D platform, a strong balance sheet, and the capabilities to execute at scale."
"Our regional business is built around a portfolio of high-impact, first- and best-in-class medicines that are driving durable, multi-year growth," said Josh Smiley, President and Chief Operating Officer of Zai Lab. "This engine provides a strong and growing financial foundation to support our long-term strategic priorities. At the same time, our dual-gateway model and partner-of-choice position enable us to consistently access high-quality innovation and advance those assets efficiently on a global stage."
Zai Lab's Dual-Engine Strategy
Zai Lab's differentiated dual-engine model is designed to drive both near-term performance and long-term global value creation. The Company's commercially profitable and scaling China business provides a strong financial foundation to invest in global innovation. Its fully integrated cross-border R&D platform enables faster, more capital-efficient development of high-quality assets, as demonstrated by Zoci's rapid advancement from first-in-human studies to global pivotal trials. Together, these two engines uniquely position Zai Lab to build a globally competitive biopharmaceutical company.
Advancing Differentiated Global Programs Across Oncology and Immunology
Zocilurtatug Pelitecan (Zoci or ZL-1310)
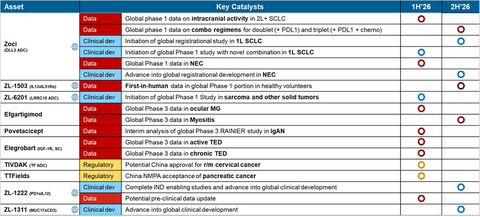
Zoci, the Company's lead global asset and a potential first- and best-in-class DLL3-targeting ADC, is expected to be in three registrational studies by the end of 2026:
- 2L/3L SCLC (small cell lung cancer): Data demonstrated a 68% overall response rate (ORR) with a favorable safety profile, including low rates of Grade 3+ adverse events and no treatment-related discontinuations at 1.6 mg/kg. A registrational Phase 3 study has been initiated.
- 1L SCLC: An ongoing Phase 1 combination study with PD-L1 ± chemotherapy is expected to inform the design of a Phase 3 study anticipated to initiate by year-end. A novel combination Phase 1 study is expected to initiate in the first half of 2026.
- NEC (neuroendocrine carcinoma): A Phase 1 study is ongoing, with results expected in the first half of 2026. A registration-enabling study is expected to initiate in the second half of 2026.
Other Global Oncology Assets
- ZL-6201: A novel LRRC15-targeting ADC designed to disrupt the tumor microenvironment by targeting tumor-associated fibroblasts (TAF), enabling potential for broad applicability across multiple solid tumors (sarcoma, breast cancer, NSCLC). Global Phase 1 initiation is expected in 1Q 2026.
- ZL-1222: A next-generation PD-1/IL-12 immunocytokine that has demonstrated strong anti-tumor activity in preclinical models, including in PD-1-sensitive and resistant settings, with an improved systemic safety profile. IND-enabling studies are expected to complete this year.
- ZL-1311: A next-generation T-cell engager (TCE) targeting MUC17, a promising and druggable antigen overexpressed in up to ~50% of gastric and gastroesophageal junction cancers. The program represents Zai Lab's first globally owned TCE and strategically expands our immuno-oncology portfolio while leveraging our established expertise in GI cancers. ZL-1311 is expected to enter global clinical development this year.
Zai Lab is building capabilities in TCEs and exploring additional immunocytokines beyond IL-12, with further details to be provided throughout the year.
ZL-1503: A Novel Dual-Targeting Approach for Atopic Dermatitis (AD)
ZL-1503 is a first-in-class bispecific antibody dual-targeting IL-13 and IL-31R designed to provide rapid itch relief and broad disease control.
- The Company anticipates reporting First-in-Human (FIH) data from healthy volunteers in the second half of 2026, paving the way for Phase 2 development in AD patients.
Key Near-Term Regional Launches to Drive Steady Growth
Today, Zai Lab has eight commercial products in China, forming a diversified and durable commercial portfolio. COBENFY is expected to launch in the first half of 2026 through a focused, high-impact commercial strategy, emphasizing physician education, real-world evidence generation, and preparation for potential NRDL inclusion in 2027. Additional near-term launches, including povetacicept and VRDN-003, are expected to add further layers of growth to the regional business. As China's regulatory and market access environment continues to evolve, Zai Lab is well positioned to deliver long-term growth.
Catalyst-Rich 2026: Granular Milestones to De-Risk Pipeline and Drive Value
2026 is expected to be a defining year for Zai Lab, with multiple high-impact milestones across its global pipeline. Key catalysts include the continued execution of Zoci's pivotal program, the advancement of multiple novel oncology and immunology assets into the clinic, several anticipated IND filings, and data readouts designed to further validate the Company's integrated R&D platform. On the regional front, the expected launch of COBENFY and continued growth of existing product franchises are expected to further strengthen the Company's financial foundation. Together, these milestones are expected to continue to de-risk the pipeline, accelerate value inflection points, and support Zai Lab's next phase of global growth.
About Zai Lab
Zai Lab Limited (NASDAQ:ZLAB, HKEX: 9688)) is an innovative, research-based, commercial-stage biopharmaceutical company based in China and the United States. We are focused on discovering, developing, and commercializing innovative products that address medical conditions with significant unmet needs in the areas of oncology, immunology, neuroscience, and infectious disease. Our goal is to leverage our competencies and resources to positively impact human health.
For additional information about Zai Lab, please visit www.zailaboratory.com or follow us at www.twitter.com/ZaiLab_Global.
Zai Lab Forward-Looking Statements
This press release contains forward-looking statements relating to our future expectations, plans, and prospects, including, without limitation, statements regarding the prospects of and plans for developing and commercializing our products and pipeline assets. These forward-looking statements may contain words such as "aim," "anticipate," "believe," "could," "estimate," "expect," "forecast," "goal," "intend," "may," "plan," "possible," "potential," "will," "would," and other similar expressions. Such statements constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are not statements of historical fact or guarantees or assurances of future performance. Forward-looking statements are based on our expectations and assumptions as of the date of this press release and are subject to inherent uncertainties, risks, and changes in circumstances that may differ materially from those contemplated by the forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including but not limited to (1) our ability to successfully commercialize and generate revenue from our approved products, (2) our ability to obtain funding for our operations and business decisions, (3) the results of our clinical and pre-clinical development of our product candidates, (4) the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approvals of our product candidates, (5) risks related to doing business in China, and (6) other factors identified in our most recent annual and quarterly reports and in other reports we have filed with the U.S. Securities and Exchange Commission. We anticipate that subsequent events and developments will cause our expectations and assumptions to change, and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, except as may be required by law. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Our SEC filings can be found on our website at www.zailaboratory.com and the SEC's website at www.sec.gov.
View source version on businesswire.com: https://www.businesswire.com/news/home/20260113130363/en/
For more information, please contact:
Zai Lab Investor Relations:
Christine Chiou / Cyan Liu
+1 (917) 886-6929 / +86 195 3130 8895
[email protected] / [email protected]
Zai Lab Media:
Shaun Maccoun / Xiaoyu Chen
+1 (857) 270-8854 / +86 185 0015 5011
[email protected] / [email protected]
